Dermatophytosis infections are caused by dermatophytes. Drug resistance and toxicity associated with long-term treatment with
conventional antifungal drugs has necessitated search for new drugs to treat fungal infections. Natural products found in plants have
been scientifically proved to avoid these side effects. The aim of this study was to formulate herbal antifungal cream containing extract of
Acalypha wilkesiana as an anti-dermatophytic preparation and evaluate its physicochemical properties, stability and efficacy of the product.
The formulated creams containing 0.5, 1 and 2% w/w of extract were subjected to stability tests using temperature variation method at
-10, 4, 30, 37 and 45oC. Freeze-thaw test, Centrifuge test, pH and exposure to UV light test were also carried out using standard method.
Efficacies of the cream formulations were determined using albino rats.
The percentage yield of the extract was (10.2%). Percentage ethanol phytochemical composition indicated that for Alkaloid it is 4.58
± 0.01%, saponins (3.10 ± 0.23%), flavonoids (1.61 ± 0.04%) and tannins (0.81 ± 0.02%). The antifungal results are in the increasing order
Microsporum audounii = Epidermophyton floccosum < M.furfur < Trichophyton mentagrophtes. Temperature stability tests carried out
indicated that the cream was very stable. Centrifuge testing indicated that there was no separation of the cream. Light testing indicated
no change in the colour and odour of the products. There was no change observed in all the test samples during the freeze-thaw testing.
Animal studies evaluation of the ethanolic formulations of the cream indicated that their efficacy against the dermatophytes is concentration
dependent and the efficacy is in the increasing order M.audounii < E.floccosum < M.furfur < T.mentagrophyte which shows that 2%
Acalypha wilkesiana cream was statistically significant (P<0.05) against all the test microorganisms.
Keywords: Dermatophytosis; Keratinization; Acalypha wilkesiana; Alkaloid, Malassezia furfur
Dermatophytosis is a skin condition caused by dermatophytes
which is fungi, and they require keratin for growth. There have
been reports by researchers that dermatophytes have the ability
to obtain nutrients from keratinized materials such as the skin,
hair and nails. Skin infection commonly called ringworm which
infects the legs, arms, beard area, scalp and the groin area has
been attributed to be caused by these dermatophytes [1].
There have been reports of the ability of Medicinal plants
to synthesize chemical compounds that are used to defend
themselves against attack from predators such as insects
and fungi [2]. Developing countries often resort to the use of
medicinal plants products to treat diseases because the products
are more affordable than purchasing modern pharmaceuticals. Acalypha wilkesiana otherwise known as copper leaf is classified
into the family of Euphorbiacaece. The genus is comprised of
about 570 species, while some are classified as weeds; others are
known to be used as ornamental plants [3]. It is a tropical plant
found in America, Africa and Asia which grows everywhere and
might have been introduced into West Africa [4]. The plant is an
evergreen shrubs having splash of colours determined by how
it is cultivated [5]. Studies carried out by Oladunmoye reported
the presence of important phytochemicals present in the leaves
of A. wilkesiana. Adesina et al., also reported that A. wilkesiana
has antibacterial and antifungal properties. Our aim for this
study was to formulate a cream with different concentrations
of A. wilkesiana extract as antidermatophyte preparations, and
evaluate their stability and physicochemical properties.
Plant materials
Leaves of Acalypha wilkesiana was collected at the Botanical
Gardens of the University of Ibadan. It was identified by the
taxonomist and sample deposited with herbarium number
MPNH/2017/1252 at the Medicinal Plants of Nigeria Herbarium
of NNMDA. The plants were air dried in the shade and pulverized
to fine-sized particles for solvent extraction processes.
Preparation of plant extracts
200g of the pulverized plant sample was extracted with
ethanol and using soxhlet extraction method. Rotary evaporator
was used to recover the solvent from the mixture.
Phytochemical constituents Determination
Simple qualitative and quantitative methods of Trease and
Evans (1989) and Sofowora (1993) were used to determine the
presence or otherwise of phytochemical constituents.
Micro-organisms
Clinical isolates of Microsporum audouinii, Epidermophyton
floccosum, Trichophyton mentagrophyte and Malassezia furfur
were obtained from Spectralab Medical and Diagnostic Services,
Sagamu, Ogun State.
Microbiological Assay
Zones of inhibition were determined using the method as
described by Irobi and Daramola (1994). The zones of inhibition
were measured in mm and recorded.
Materials for Emulsion Formulation
All oil soluble substances were placed in a stainless steel
container and heated to between 70-75oC. All water-soluble
substances were placed in another stainless steel container and
heated to the same temperature. The oil phase was then added to
the aqueous phase slowly with stirring. Heating was continued
at the same temperature for about 10-15 minutes. The coarse
emulsions formed were then cooled to about 35oC gradually. The
emulsions were allowed to stay at room temperature for twelve
hours and then homogenized with the aid of a mechanical stirrer.
The samples were then poured into labeled containers. Emulsions
containing 0.5%, 1% and 2%, of Acalypha wilkesiana ethanolic
extracts were produced. The prepared herbal emulsions were
then vigorously homogenized.
Stability Tests for the formulated emulsion
Stability tests were carried out on the emulsions following the
methods as described by Cannel (1992) whereby the temperature
variation tests include storing the samples at -10oC, 4oC, 30oC,
37oC and 45oC were carried out. All observations including pH,
colour and odour were noted and recorded. The creams were
made to pass through Freeze thaw cycles testing which involves
making the samples pass through three cycles of temperature
testing by placing the samples at -10oC for 24 hours and then at
room temperature for 24 hours. The creams were made to pass
through centrifuge testing whereby the samples were heated to
50oC and they were then centrifuged for thirty minutes at 2000,
2500, 3000 and 4000 rpm. They were then inspected for signs to
determine if the dispersed phase of the emulsion has separated
and risen to the top. The creams were made to pass through light
testing whereby they were placed in test tubes and also in the
actual package. They were then put in the window where direct
sun rays fell on them. This method is used to determine the
sensitivity of the emulsions to the Ultra Violet radiation.
In vivo antidermatophytic activity
Ethical statement: The experimental procedures complied
with University of Ibadan ethics committee in line with approval
number UI-ACUREC/App/12/2016/06.
Laboratory Animals: Albino rats weighing between 150-
200g were kept in cages with access to water and feed. They were
left in this environment for two [6] weeks to acclimatize [7].
Selection and grouping of animals: The animals were
randomly allocated to six [8] groups (5 rats/group) such that the
difference in average weight did not exceed 5g. Each animal was
used once in the experiment. The rats were then inoculated with
the dermatophytes. One week after inoculation of the animals
with the dermatophytes, the inoculated skin area of 2cm2 were
treated with the plants extracts and the formulated emulsions
with plants extracts for seven days. They were euthanized at the
end of seven days.
Histopathological studies: 2cm2 skin areas were cut and
put in 10% formalin for histopathological analysis. Skin biopsy
samples were examined for presence of fungal hyphae, hair
follicles, sebaceous gland, inflammation and tissue destruction
using light microscope [9].
Statistical analysis of Data: ANOVA was used followed by
tukeys post hoc analysis. Data is reported as mean ± SEM. P ≤
0.05 was considered significant. Also epidermal thickness and
keratin layer were obtained with the aid of calibrated Toupview®
software (Table 1).
Antifungal activities
The results obtained for the antifungal activities indicated
that the ethanol extract of the leaves of the plants was very active
against the test microorganisms as shown in Figure 1. At 10μm/
mL, M.furfur had the highest activity with zone of inhibition of
9mm followed by T.mentagrophyte (7mm) and then M.aoudinin
(5mm). There was no activity recorded against E.floccossum.
At 100μm/mL, the activity of M.furfur, T.mentagrophyte and
M.aoudinin remain the same but activity was noticed for
E.floccossum (4mm) at this concentration. There was marked activity of the extract against the dermatophytes at 1000μm/
mL. Activity against M.furfur was 11mm > T.mentagrophyte =
E.floccossum (9mm) > M.aoudinin (8mm). Activity of the extract at
10000 μm/mL was M.furfur (14mm) > T.mentagrophyte (12mm)
> E.floccossum (10mm) > M.aoudinin (9mm). The results obtained
indicated that the activity against the dermatophytes are in the
order M.furfur > T.mentagrophyte > E.floccossum > M.aoudinin.
Temperature Stability Testing
The pH of the samples at two weeks of test was slightly
lower compared to the pH at day 1 of production. Colour and
odour remain stable across the test temperatures except at 45oC
where there are detectable changes in the colour and odour of
the samples. At four weeks of test, the pH remained stable at all
the test temperatures. There was no colour or odour change.
After eight weeks of test; no changes were detected in the pH,
colour and odour of the test samples. At twelve weeks, there was
slight decrease in the pH of the samples and also slight changes
in the colour and odour. At sixteen weeks of test, there were no
significant changes in pH, colour and odour compared to what
was observed at twelve weeks of test.
Centrifuge Testing
No phase separation was detected in all the test samples at
2000, 2500, 3000 and 4000 rpm. Also, during light and cycle
testing, there were no changes noticed (Table 2).
Histopathology of skin tissues
Tissues were examined for the presence of fungal elements;
inflammation, Fungal hyphae, loss of hair follicles, absence of
sebaceous gland and discontinuity/Tissue destruction as well as
dekeratinisation and epidermal thickness.
Animal studies using Acalypha wilkesiana ethanol extracts against the dermatophytes
The untreated control group indicated heavy infection of the hairs by the dermatophytes (Figures 2,5 and 6). Same
goes for the group treated with the emulsion alone, the group
treated with 0.5% Acalypha wilkesiana extract alone and the
group treated with 0.5% Acalypha wilkesiana extract emulsion
was not statistically different from one another (Figure 3). The
values obtained for 1% Acalypha wilkesiana extract alone and
1% Acalypha wilkesiana emulsion indicated slight efficacy by the
formulations (Figure 4). Comparatively, 2% Acalypha wilkesiana
extract alone and 2% Acalypha wilkesiana extract emulsion
showed moderate mycological efficacy. The standard drug 1%
clotrimazole cream demonstrated high mycological efficacy in
preventing attack by the dermatophyte on the keratin layer of the
hair. Statistically, there is significant difference in keratinization
between the groups as determined by one way Anover.
There was significant infection as indicated by the result
obtained for the epidermal thickness of untreated group. The
values obtained for emulsion alone, 0.5% Acalypha wilkesiana
extract, 0.5% Acalypha wilkesiana extract emulsion, 1%Acalypha
wilkesiana extract and 1% Acalypha wilkesiana emulsion
in reducing the epidermal thickness cannot be said to be
significant compared with the untreated control. However, the
values obtained for 2% Acalypha wilkesiana extract alone and
2% Acalypha wilkesiana emulsion with extract showed indication
that there was moderate reduction in the epidermal thickness
thereby suggesting that the formulations are mycologically
effective against the dermatophyte compared with the values
obtained for the standard drug. The statistical evaluation
indicated that there is significance difference in epidermal
thickness between groups.
Angkhana Inta et al., , reported that plants extracts were used
in the treatment of different ailments and have been known to
inhibit microorganisms. Phenolic compounds of natural origin
have been reported to play an important role in the management
and treatment of diseases [10].
The chemical constituents of the plant crude extract showed
that it was rich in alkaloids, saponins, tannins and flavonoids
which have been known to exhibit medicinal activities [11]. Our
findings are in agreement with works carried out by Oladunmoye,
2006 and Ezekiel et al., [12].
The results show that the crude ethanol extract of
Acalypha wilkesiana possesses antifungal activities against
the dermatophytes, thus confirming the folkloric use of the
plant. This indicated that the plant extract may be effectively
used in the management of dermatophytosis. The extract had
activity against T.mentagrophyte, M.aoudinin and M. furfur at
all concentrations with zones of inhibition in the range of 5-14
mm except against E.flocossum at 10μg/mL. Adesina et al., [13]
confirmed this observation. This activity may be attributed to
the active components which are enhanced in the presence of
ethanol [14-18].
The temperature stability testing carried out on the
formulation indicated that the product is very stable, though
some changes were noticed at elevated temperature of 45oC. The pH was stable but there was a noticeable change in odour
and colour of the product at 45oC. This could be due to the fact
that the elevated temperature degenerate the components of
the products. There was no significant change during cycle and
centrifuge tests. Also there was no change in colour during the
light test.
Evaluation of the efficacy of the antidermatophyte cream using
albino rat’s model indicated that 2% formulation was as effective
as the control drug. Inflammatory response in the infected rats
which is largely composed of neutrophilis in the early phases was
reversed with treatment with the formulation (Figure 5). In most
cases, few fungal elements were detected in the stratum cornea
of skin from infected animals. Also it was observed that there are
sebaceous glands compared to the untreated group. Our data
using the epidermal thickness values showed that the cream is
most active against T.mentagrophyte (37.01 ± 0.39) > M.furfur
(35.83 ± 0.57) > E.flocossum (33.65 ± 0.74) > M.aoudinin (31.01 ±
0.62) which show that the formulation is statistically significant
(P<0.05) against all the test microorganisms.
This study shows that Acalypha wilkesiana ethanol extract has
high potential as an antidermatophyte agent when formulated
as cream for topical application. This explains the folkloric
use of the medicinal plant. Among the prepared formulations,
2% formulation showed highest activity against all the
dermatophytes. The formulations showed acceptable physical
properties and were stable during the accelerated stability test.

 Table 1: Percentage phytochemical composition.
Table 1: Percentage phytochemical composition. 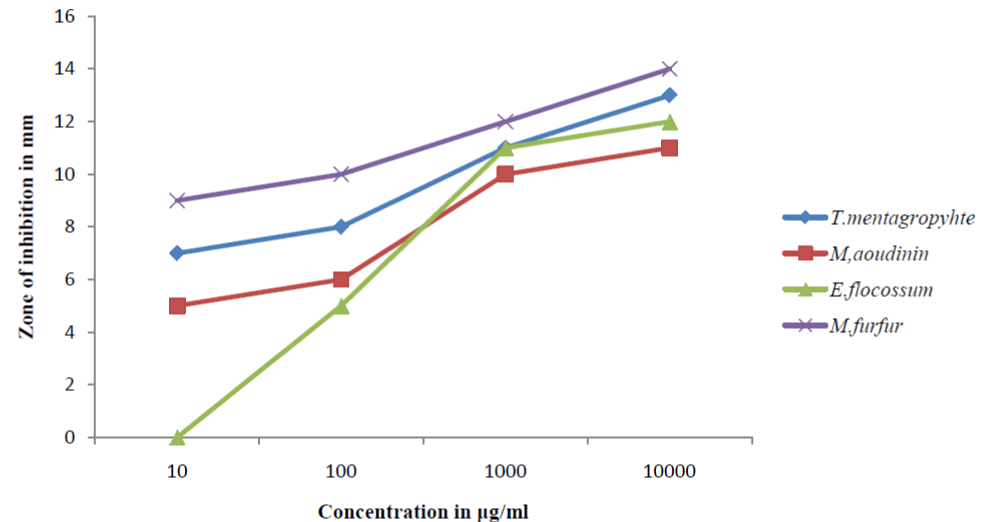 Figure 1: Antifungal Screening of Acalypha wilkesiana ethanol extracts.
Figure 1: Antifungal Screening of Acalypha wilkesiana ethanol extracts. 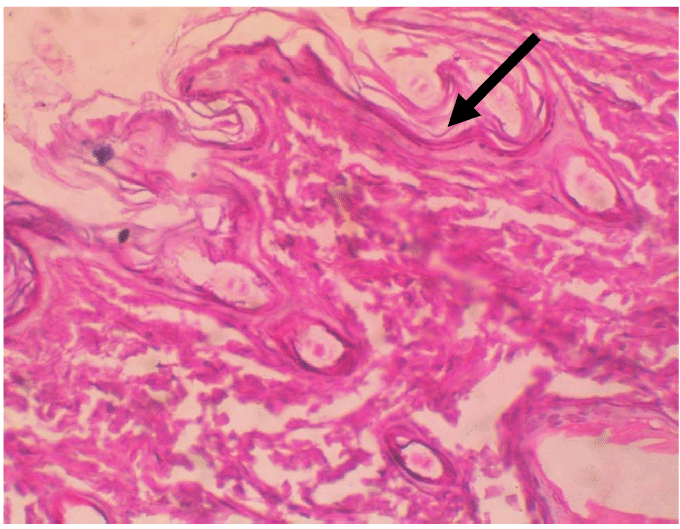 Figure 2: Tissue destruction by the dermatophyte H & E stain x100.
Figure 2: Tissue destruction by the dermatophyte H & E stain x100. 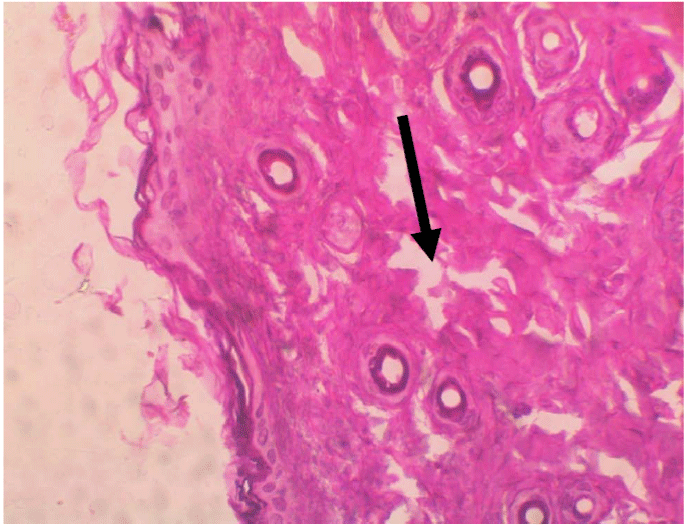 Figure 3: Discontinuity of the skin structure H & E stain x100.
Figure 3: Discontinuity of the skin structure H & E stain x100. 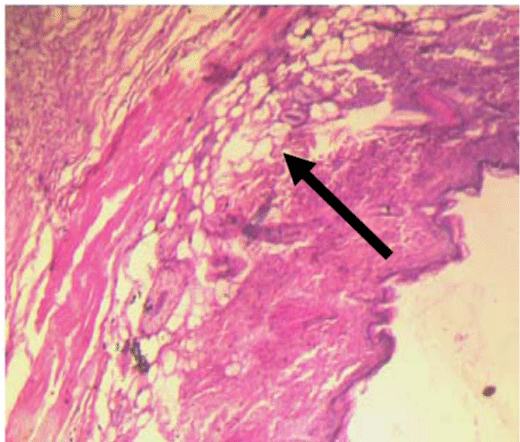 Figure 4: Inflammation of the skin tissues H & E stain x100.
Figure 4: Inflammation of the skin tissues H & E stain x100. 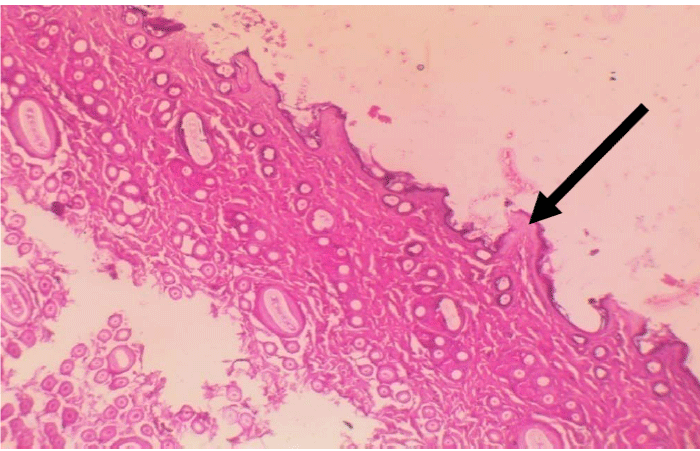 Figure 5: Increase in epidermal thickness.
Figure 5: Increase in epidermal thickness. 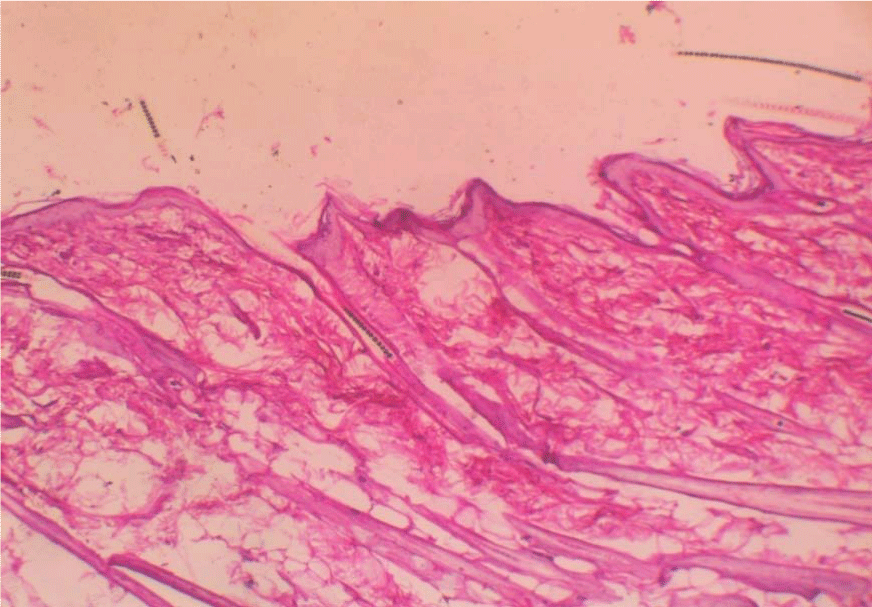 Figure 6: Invasion by dermatophyte hyphae H & E stain x100.
Figure 6: Invasion by dermatophyte hyphae H & E stain x100.