|
Lymph Node Grade |
||||
Histopathologic Diagnosis |
LN0-1 |
LN2 |
LN3 |
LN4 |
ND |
CTCL (n= 30)* |
0 |
0 |
6 |
24 |
0 |
Clonality (Pos/Neg/ND) |
0 |
0 |
3/0/3 |
10/2/12 |
0 |
FC (Pos/Neg) |
0 |
0 |
3/3 |
19/5 |
0 |
DL/Other† (n= 42) |
17 |
10 |
7 |
0 |
8‡ |
Clonality (Pos/Neg/ND) |
6/6/5 |
1/7/2 |
4/1/2 |
0 |
2/2/4 |
FC (Pos/Neg) |
2/15 |
3/7 |
3/4 |
0 |
1/7 |
All excised LNs (n= 72) |
17 |
10 |
13 |
24 |
8 |
Clonality (Pos/Neg/ND) |
6/6/5 |
1/7/2 |
7/1/5 |
10/2/12 |
2/2/4 |
FC (Pos/Neg) |
2/15 |
3/7 |
6/7 |
19/5 |
1/7 |
Abbreviations: LN, lymph node; CTCL, cutaneous T cell lymphoma; DL, dermatopathic lymphadenopathy, FC, flow cytometry; ND, not done. |
|||||
JSM Clinical Pathology
-
Research ArticleFlow Cytometric Criteria for Lymph Node Ratings and Staging of Mycosis Fungoides and Sézary SyndromeEric C. Vonderheid1*, J. Steve Hou2, and Robert Bigler31Sydney Kimmel Comprehensive Cancer Center, Johns Hopkins Medical Institutions, USA
2Department of Pathology, Drexel University College of Medicine, USA
3Hematologist, private practice, Former Director Flow Cytometry Laboratory, Hahnemann University Hospital, Philadelphia, USA*Corresponding author: Eric Vonderheid, M.D., 37580 S. Desert Sun Drive, SaddleBrooke, AZ 85739 USA. Phone: +1-520-825-2699; Email: evonder1@jhmi.eduSubmitted: 14 November 2018; Accepted: 15 May 2019; Published: 16 May 2019 -
Objectives: Determine flow criteria corresponding to LN4 histopathologic grade used for clinical staging of cutaneous T cell lymphoma (CTCL).Materials and Methods: Flow cytometry using primarily a panel of single antibodies was performed on 78 excised lymph nodes (LNs) from 72 patients with CTCL. Correlation with histopathologic grade was available for 64 specimens. Fifty-two LNs obtained at initial staging were used for prognostic correlations. Seventeen additional LNs were studied by fine needle aspiration (FNA).Results: Several flow criteria including CD4/CD8 ≥ 30, all CD7+ cells ≤ 20%, CD7/CD4 ≤ 0.4, CD71+ cells ≥ 20% and CD4/CD19 ≥ 8 had mean disease-specific survival rates comparable to that observed for patients with LN4 grade. However, less than 50% of LN4 nodes had these criteria. In a smaller subset of patients studied mostly by FNA, direct measurement of CD4+CD7- and/or CD4+CD26- cells provided quantitative data that also identified patients with survival rates similar to LN4 grade. The percentage of CD4+CD26- cells was typically higher than the percentage of CD4+CD7- cells.Discussion: FNA is useful to assess enlarged peripheral LNs for involvement with CTCL. In conjunction with FNA, we propose that the maximum percentage of either CD4+CD7- and CD4+CD26- lymphocyte subsets ≥ 50% plus additional confirmatory evidence of involvement be used to define N3 rating and perforce sub-stage IVA2. Additional studies are required to confirm these results and determine the role that FNA vis-à-vis excisional biopsy plays in the evaluation of patients with CTCL.Keywords: Sézary; Mycosis fungoides; Lymphoma; CD26; CD7; CD71; Staging
-
LN: lymph node; CTCL: cutaneous T cell lymphoma; MF: mycosis fungoides; FC: flow cytometry; FNA: fine needle aspiration; NCI-VA: National Cancer Institute–Veterans Administration; ISCL/EORTC: International Society for Cutaneous Lymphomas/European Organization for Research Treatment of Cancer; TCR: T cell receptor; PCR: polymerase chain reaction; SEM: standard error of the mean; ANOVA: analysis of variance test; K-W: Kruskal-Wallis test; DSS: disease-specific survivalEnlargement of peripheral lymph nodes (LNs) is associated with a poor prognosis in patients with mycosis fungoides (MF) and Sézary syndrome (SS), the most common presentations of cutaneous T cell lymphoma (CTCL) [1,21]. LN enlargement is usually the result of dermatopathic lymphadenopathy (paracortical T zone hyperplasia with melanophages) combined with variable degrees of infiltration by neoplastic T cells.1Atypical/neoplastic cells defined as (1) small or large cells with irregularly folded, convoluted nuclear outlines, a cerebriform appearance, and nuclear hyperchromasia; (2) immunoblastic or transformed lymphoid cells with slightly basophilic or clear cytoplasm and large nuclei with a vesicular chromatin pattern and prominent nucleoli; and (3) large cells with multiple or multilobed nuclei and cells closely resembling Reed-Sternberg cells.For clinical staging, the ISCL/EORTC recommends that peripheral LNs that are 1.5 cm or larger in diameter be excised and examined for involvement by routine histopathology 2[3]. Ancillary studies such as molecular genetic studies to detect T cell clonality (ideally the same clone as in involved skin) or flow cytometry (FC) to detect lymphocytes with aberrant phenotypes are helpful to confirm involvement and evaluate LNs that are not overtly involved with lymphoma.2B2 defined as absolute Sézary cell count ≥ 1.0/μL or CD4/CD8 ratio ≥ 10 plus evidence of T cell clone or T cells with abnormal phenotype in the blood.However, only peripheral LNs that are partially or completely effaced by neoplastic T cells, i.e., histopathologic grades III/IV in the Dutch scoring system [4] or the LN4 histopathologic grade in the National Cancer Institute–Veterans Administration (NCIVA) classification [5], are currently utilized for clinical staging. This defines the N3 lymph node rating and perforce stage IVA2 (T1-4N3B0-2M0) [3]. Almost all effaced LNs have dominant T cell clones that can be demonstrated by either Southern blot or PCRbased methods [6-10]. Effaced LNs usually occur in the setting of clinically advanced CTCL and are associated with a significantly worse prognosis compared to non-effaced LN patterns [2,11].In the NCI-VA classification scheme, non-effaced LNs are scored between LN0 to LN3 grades based on numbers of atypical/neoplastic cells in parafollicular zones3: LN0: no atypical cells; LN1: isolated atypical cells; LN2: small clusters of atypical cells; and LN3: large clusters or sheets of atypical cells without effacement of nodal architecture. The detection of T cell clones in non-effaced LNs indentifies patients with a worse prognosis compared to patients without a detectable clone [6-10,12], and it has been suggested that LN3 grade nodes with evidence of a clone, particularly when demonstrated with the Southern blot method [6-8], should be considered comparable to LN4 nodes for use in clinical staging. An N2b node rating is designated for such cases, but currently it is not considered comparable to N3 rating for staging of CTCL patients [3].3Of interest, the latter patient’s disease subsequently evolved into SS, but at the time of the initial LN biopsy, the absolute Sézary cell count was 113 K/μL, the CD4/CD8 ratio was 66%/10% and Southern blot analysis showed no evidence of a clone in the blood.We and others reported that fine needle aspiration (FNA) with evaluation of the sample by cytopathology, PCR analysis of the T cell receptor (TCR) gene and FC provides an additional means to assess LNs from patients with CTCL [13-15]. Specifically, we observed a strong correlation between the grade I to IV cytopathologic score based on numbers of atypical cerebriform lymphocytes obtained by FNA and the NCI-VA score for 11 excised nodal specimens [13]. However, although involvement of LNs by CTCL (or other lymphomas) can be established by FNA, the method does not reliably determine the degree of effacement of nodal architecture which is a prerequisite for clinical staging, i.e. N3 rating. The purpose of this study is to review our experience with FC obtained on LNs from patients with MF/SS and determine if a criterion based on FC for N3 node ratings can be established for use with FNA samples.FC was performed on a portion of 78 excised peripheral LN from 72 patients with CTCL (32 diagnosed with MF and 40 with erythrodermic CTCL (E-CTCL) which includes 21 patients with SS (B2 blood rating4) and 18 with erythrodermic MF (E-MF) as defined by ISCL/EORTC criteria [3]. The number of Sézary cells per 100 lymphocytes on blood smears was visually counted by one experienced technician as previously reported [16], and the absolute Sézary cell count calculated from absolute lymphocyte count determined from the concurrent WBC and leukocyte differential count. The magnitude of LN involvement (LN grade) was assessed using the NCI-VA classification scheme [5]. In addition, the neoplastic cells of overtly involved LNs were classified according to small, mixed small-large or large cell morphology as previously described [11].4LNs with neoplastic cells that did not express CD4 (one LN4, one LN0-2), coexpressed CD4 and CD8 (one LN4 node) or were not studied for CD7 (one LN4 node) are excluded.Two LN specimens (pathologic diagnosis in parentheses) were obtained from one patient with MF and large cell transformation in the skin (both dermatopathic lymphadenopathy), 2 patients with SS whose disease progressively worsened despite treatment (dermatopathic lymphadenopathy and CTCL for one patient and small cell and mixed small-large cell lymphoma pattern for the other patient), and 1 patient with E-MF that progressed to SS (both dermatopathic lymphadenopathy). Three LN specimens were acquired from a patient with plaque phase MF (focal involvement with MF) who progressed to the tumor phase (dermatopathic lymphadenopathy), and subsequently leukemic involvement (diffuse involvement with lymphoma). For prognostic correlations, only LNs that were obtained at the time of initial staging of 52 patients were used.Single color flow immunophenotyping was performed on 52 excised LNs studied before 1997 as previously described5 [17]. The antibodies in the panel reacted against T-cell markers (CD2, CD3, CD4, CD5, CD7, CD8), B-cell markers (CD19, CD20, kappa and lambda light chains), natural killer (NK) cell markers (CD16, CD57), activation/proliferation markers (HLA-DR, CD25, CD71) and CD10. The ratios of CD4/CD8 and kappa/lambda were provided by the laboratory. For 26 excised LN specimens studied after 1997 and specimens obtained by FNA, two or three color immunophenotyping that used CD45 and side scatter to define the lymphocyte gate provided measurement of the CD4+CD7- and CD4+CD26- lymphocytes as well as expression of CD16+CD56+ NK cells. FNA was also directly obtained on 4 excised LNs for comparison with the portion submitted for FC; the latter specimen was used for analysis [13]. The ratios of CD7/CD4 and (CD7-CD8)/CD4 were calculated to estimate the expression level of CD7 on CD4+ cells. Although both parameters correlated well with available direct measurements of CD4+CD7- cells (rho= -0.952 and rho= -0.948, respectively, P= 0.01), CD7/CD4 ratio was chosen for comparative analysis. Southern blot analysis of the TCR beta chain or PCR analysis of the TCR gamma chain for evidence of a T-cell clone was performed on 45 excised LNs (40 of the initial LNs). The presence of either cells expressing an abnormal immunophenotype or a T cell clone was defined as a positive ancillary study.5We assume that LNs with histopathologic evidence of CTCL would be abnormal by cytopathology and/or special studies. However, we acknowledge that focal involvement might be missed by FNA.FNA of enlarged peripheral LNs was performed directly on 18 patients and submitted for cytopathology and ancillary studies. The clinical diagnosis was MF for 6 patients (5 at tumor phase, 1 at patch phase) and E-CTCL for 12 patients (9 with SS, 3 E-MF). The cell yield was inadequate for one sample from a patient with SS and sufficient to allow measurement of CD4+CD7- and CD4+CD26- lymphocytes on 17 and 16 specimens, respectively. CD19+ cells with κ/λ ratio were measured on 17 and 14 samples, respectively. CD2, CD5, CD25 and HLA-DR were measured on 8 samples and will not be discussed further. PCR for T cell clonality was performed on 16 of 18 cases.Results of laboratory studies were given as mean values ±1 standard error of the mean (SEM) and/or median value with a range. Fisher’s and Pearson’s chi-square exact tests were used to test categorical data. Welch’s t-test, which does not assume equal variances, was used to compare mean values of two independent samples. For 3 groups, mean values were compared using oneway analysis of variance (ANOVA) together with the Games- Howell post hoc test when differences were significant. The nonparametric Kruskal-Wallis (K-W) test was also used to test for differences in median values. Spearman’s correlation coefficient was used to test for significant correlations. The Cox Proportional Hazards model and Kaplan–Meier estimates of survivor function were used for survival analysis. Deaths attributed to CTCL or its treatment defined disease-specific survival (DSS) in these models. The statistic -2 log L was used to compare alternative Cox models (the lower the value of -2 log L, the better the fit in the model) [18]. Statistical software used in the study were SYSTAT10 and SPSS 13.0 for Windows, SPSS, Inc. (Chicago, IL) and StatXact-3, Cytel, Inc. (Cambridge, MA).The relationship between histopathologic diagnosis and clinical diagnosis at the time of biopsy is given in Supplemental Table 1. Of 78 excised LNs, 33 (42%) nodes had histopathologic evidence of CTCL and the remaining nodes showed reactive changes, typically dermatopathic lymphadenopathy. Involvement was more likely to be present in LNs from patients with SS (17/24 nodes = 71%) than patients with MF (14/35 nodes = 40%) or E-MF (2/18 nodes = 11%, P< 0.001). The morphologic pattern of neoplastic cells in involved LNs of SS and MF was not significantly different (P= 0.426).NCI-VA histopathologic grades and results of FC and molecular genetics were recorded for 72 excised LNs obtained for clinical staging (Table 1 and Supplemental Table 2). Of 30 LNs diagnosed with CTCL, 6 had paracortical expansion with neoplastic cells while retaining germinal centers (LN3) and 24 had partial or complete effacement of nodal architecture (LN4). Of the 42 non-involved LNs, 7 were graded as LN3 and none were LN4. Thus, LN3 grade was recorded in 20% (6/30) and 21% (7/34) of LNs diagnosed as involved or not involved with CTCL, respectively.
-
 Table 1:Histopathologic diagnosis and ancillary studies obtained on 72 initially excised lymph nodes from patients with mycosis fungoides and
Sézary syndrome. View Table
Table 1:Histopathologic diagnosis and ancillary studies obtained on 72 initially excised lymph nodes from patients with mycosis fungoides and
Sézary syndrome. View Table
The prognostic implications of histopathologic grading of initial LNs are shown in Table 2. As expected, histopathologic involvement signified a worse prognosis than non-involvement. The DSS curves associated with LN0-1 and LN2 nodes were nearly identical and therefore were combined for subsequent analysis (LN0-2). Overall, the mean DSS of patients with LN0-2 nodes (14.42 years) was significantly better than LN3 nodes (7.08 years; P= 0.026) and LN3 nodes were better than LN4 nodes (2.32 years; P= 0.008, Supplemental Figure 1). Of note, the difference in DSS for patients with involved LNs classified by morphologic appearance was not significant (P= 0.523).-
 Table 2:Lymph node (LN) ratings of initial specimens obtained for staging based on National Cancer Institute-Veterans Administration histopathologic
grades with or without neoplastic cells by ancillary study. View Table
Table 2:Lymph node (LN) ratings of initial specimens obtained for staging based on National Cancer Institute-Veterans Administration histopathologic
grades with or without neoplastic cells by ancillary study. View Table
Finally, when tested in the Cox model with age at biopsy entered as a covariate, both clinical diagnosis and presence of histopathologic involvement were significantly associated with deaths from CTCL as the endpoint (-2 log L changed from 285.89 to 245.23). If LN grades (LN0-2, LN3 and LN4) were substituted for histopathologic involvement in the model, the fit with outcome improved only slightly (-2 log L, 244.45).Of the 47 excised initial LNs studied by single antibodies, 13 (28%) had cells that had a diminished expression of CD7 consistent with an abnormal T cell population. One specimen had in addition loss of CD2, one had loss of CD3 and CD4 and one had co-expression of CD4 and CD8. The remaining 34 LNs were considered to have a normal phenotype. However, 12 of these specimens had CD4/CD8 ratios ≥ 10 (range, 10.8 to 42.7) and 2 expressed CD10.Of the 25 LNs studied with combinations of antibodies, 16 (64%) had cells with an abnormal phenotype. Diminished CD7 expression occurred in 9 specimens as the only abnormality or was combined with loss of CD4, CD5 or co-expression with CD10 (one case each). Four of the remaining LNs had cells expressing a CD3dim phenotype (two also loss of CD26), one had CD2- cells, one had CD3-CD30+ cells and one with CD7dimCD26- cells. Altogether, of initial LNs scored for LN grades, cells with an abnormal phenotype were detected by FC in 5 of 27 (19%) LN0-2 nodes, 6 of 13 (46%) LN3 nodes and 19 of 24 (79%) LN4 nodes (1 of 8 LNs not scored). In the Cox model with age and LN grades as covariates, evidence of cells with an abnormal phenotype was not a significant variable.Of the 43 LNs studied for clonality, a T cell clone was detected by Southern blot analysis in 7 initial LNs and by PCR in 19 nodes. The presence of a clone signified a significantly worse prognosis (Mean DSS, 5.90 years ± 0.84, 5-year 57%) compared to patients without a clone (16.55 years ± 2.28, 5-year 71%, P= 0.014). In terms of LN grades, a clone was present in 7 of 20 (35%) LN0-2 nodes, 7 of 8 (88%) LN3 nodes and 10 of 12 (83%) LN4 nodes. Although the presence of a clone on 7 LN0-2 nodes signified a worse prognosis (Mean DDS, 7.61 years ± 1.62) compared to 13 LNs without a detectable clone (Mean DDS, 17.51 years ± 2.54), the difference in survival curves was not statistically significant (P= 0.163). In the Cox model with age and LN grades as covariates, the presence of clonality was not a significant variable.Altogether a positive ancillary study occurred in 43 of 72 (60%) initial LNs overall (Table 2). The DSS for patients with a positive ancillary study (mean 4.99 years ± 0.68) was significantly shorter than patients with a negative special study (mean 11.85 years ± 1.86; P= 0.010). However, in the Cox model with age and LN grades as covariates, a positive ancillary study was not a significant variable.It should be noted that of 29 patients with a negative ancillary study, 5 were diagnosed to have LN involvement by histopathology (LN3 grade: 2 patients; LN4 grade: 3 patients). Two patients with effaced LN4 nodes had SS. Both had absolute Sézary cell counts exceeding 1.0 K/μL and T cell clone in the blood shown by molecular genetic and chromosome analysis. The LN of one patient showed a small cell pattern with decreased expression of CD7 by immunohistochemistry even though CD7 was expressed on the majority of cells studied by FC (74% CD4+, 70% CD7+). The LN from the other patient with SS showed a mixed small-large cell pattern with patchy positivity for CD7 by immunohistochemistry and 98% CD4+, 72% CD7+ by FC. Subsequent investigations showed that the neoplastic cells of this patient also expressed FoxP3 [19]. The third patient had diffuse effacement of the LN with large cells and aneuploidy demonstrated by cytophotometry, results consistent with transformed MF. FC showed 53% CD4+ and 53% CD7+ cells. Immunohistochemistry and molecular genetic analysis were not performed. The two patients with LN3 nodes had focal subcapsular involvement of the LNs, one with large cells that expressed a CD4-CD8-CD7- phenotype by immunohistochemistry, the other with a mixed small-large pattern not further characterized by immunohistochemistry. It is therefore likely that an ancillary study might have been positive in some of these patients if modern FC and molecular genetic methods that are more sensitive to detect small populations of neoplastic cells than methods used in this study.The results of FC according to LN grade of initial LNs are shown in Supplemental Table 2. Several findings on FC correlated significantly with prognosis in the Cox model with patients’ age as the only covariate. These included a high CD4/CD8 ratio (P= 0.020) due mainly to low CD8+ cells (P= 0.020) rather than high CD4+ cells (P= 0.119). When categorized as CD4/CD8 ≥ 10, the level of significance changed to P= 0.003 and this remained significant in the model with LN grade added as a covariate.In addition, given that CD7 is often lost by neoplastic T cells, [17,20-22] it was not surprising that low percentage of CD7+ cells (P= 0.002) and calculated CD7/CD4 ratio (P= 0.004) for all patients and direct measurement of CD4+CD7- cells in a smaller cohort (P= 0.025) also had prognostic importance with patients’ age in the model. However, these parameters no longer retained significance with age and LN grade as covariates.Also, the percentage of cells expressing the transferrin receptor CD71 was significant (P= 0.003) as were the calculated ratios of CD71/CD3 (P= 0.044) and CD71/CD5 (P= 0.017); CD71/ CD4 ratio was nearly significant (P= 0.084). Of interest, other activation markers CD25 (P= 0.857) nor HLA-DR (P= 0.486) were not significantly associated with survival. Again, CD71 no longer was significantly associated with survival with age and LN grades as covariates.Lastly, we reasoned that the calculated CD4/CD19 ratio might reflect the degree of nodal effacement and indeed this parameter was significant with patients’ age in the Cox model (P= 0.047) but not with LN grade (P= 0.941).We first plotted various flow parameters significantly associated with prognosis from all 54 excised LNs graded as LN0-2, LN3 and LN4 with the intent of identifying thresholds that might differentiate LN4 from LN0-3 nodes. Considerable overlap with LN3 nodes was observed for each parameter such that potential diagnostic thresholds for LN4 define only a small proportion of LN4 nodes. For example, a threshold for CD4/CD8 set at ≥ 30 identified 7 of 24 LN4 nodes, 2 of 15 LN3 nodes and none of 28 LN0-2 nodes (Supplemental Figure 2). This criterion would correctly classify only 29% of LN4 node and misclassify 5% of LN0-3 nodes. Somewhat better results were achieved with thresholds using CD7+ cells < 15% (9 of 25 LN4 or 36% positive versus 1 of 43 LN0-3 or 2% misclassified) and CD4/CD19 ratio ≥ 10 (6 of 25 LN4 or 24% positive versus none of 43 LN0-3 misclassified; Supplemental Figures 3 and 4).An alternative strategy was to define flow criteria that have mean DSS rates similar to that of 24 LN4 nodes, i.e., within the 95% confidence interval for mean DSS of LN4 nodes (1.32 to 3.32 years). Only initial LNs that might be expected to show evidence of neoplastic cells by FNA were included in this analysis. An example of this approach for CD4/CD8 ratio as a criterion is shown in Figure 1 and Supplemental Table 3. As the CD4/CD8 ratio is increased from ≥ 10 to ≥ 30, the estimated mean DSS for patients becomes shorter, and at CD4/CD8 ≥ 30, the mean DSS of 2.62 years was within the 95% confidence interval for LN4 nodes. Also, the 5-year DSS rate (22%) approximated that for LN4 nodes (17%). However, although none of the LN0-2 nodes had a CD4/CD8 ≥ 30, only 29% of LN4 nodes were positive for this criterion. In a similar way, other flow criteria were evaluated as potential surrogates for LN4 (Table 3). As with CD4/CD8 ratio, these criteria identified only a minority of LN4 nodes.-
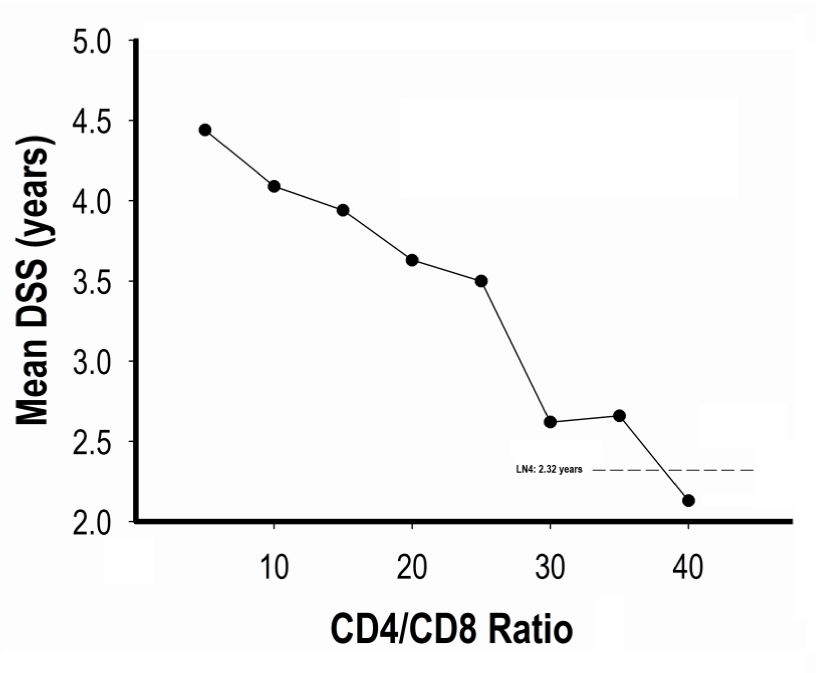 Figure 1: Plot of estimated mean disease-specific survival of patients
with mycosis fungoides and erythrodermic cutaneous T cell lymphoma
according to CD4/CD8 ratios in lymph nodes obtained at the time of
clinical staging. The dashed line shows the mean DSS of LN4 graded
lymph nodes. View Figure
Figure 1: Plot of estimated mean disease-specific survival of patients
with mycosis fungoides and erythrodermic cutaneous T cell lymphoma
according to CD4/CD8 ratios in lymph nodes obtained at the time of
clinical staging. The dashed line shows the mean DSS of LN4 graded
lymph nodes. View Figure
-
 Table 3:Flow criteria with estimated mean disease-specific survival (DSS) rates similar to patients with National Cancer Institute-Veterans
Administration histopathologic grade 4 lymph nodes. View Table
Table 3:Flow criteria with estimated mean disease-specific survival (DSS) rates similar to patients with National Cancer Institute-Veterans
Administration histopathologic grade 4 lymph nodes. View Table
Another possibility involves measuring the percentage of CD4+CD7- and CD4+CD26- lymphocytes in LNs similar to what has been proposed for the blood in CTCL. [20-25] Because CD7 and to a lesser extent CD26 may be variably expressed by neoplastic cells, it has been proposed that both CD4+CD7- or CD4+CD26- populations be measured and that the maximum percentage of either one (herein called maxiCD4+CD7-/26- percentage) be used to estimate neoplastic cell numbers in the blood. [26,27] Of the excised LNs in this series, CD4+CD7- and CD4+CD26- were measured together for only 6 graded LNs. The 2 LN0-2 nodes had maxiCD4+CD7-/26- percentage < 30% whereas both LN3 and 1 of the 2 LN4 nodes had values exceeding 40%. The exception was a LN diffusely involved with a transformed CD3-CD4+CD30+ large cell lymphoma that expressed both CD7 and CD26 and an identical T cell clone that was detected in erythrodermic skin of a patient with E-MF.FNA showed cytopathologic evidence of neoplastic cells in 12 of 17 LNs that had adequate sampling. One additional LN from a patient with tumor phase MF without abnormal cells by cytopathology nor FC had evidence of a T cell clone that was also in the skin and blood; this specimen was categorized to be minimally involved with disease. The results of FC for 13 LNs with and 4 LNs without evidence of neoplastic involvement by FNA are shown in Table 4. Of interest, 3 patients with typical SS had no detectable T cell clone in the LN specimen by PCR despite having abnormal cells by cytopathology and CD4/CD8 ≥ 10 and high percentages of CD4+CD26- cells (58%, 79% and 93%) by FC. In their concurrent blood sample, a clone was also not detected by PCR despite all demonstrating high Sézary cell counts, CD4/CD8 ≥ 10 and high percentage of CD4+CD26- cells. A chromosomallyabnormal clone was demonstrated in 2 patients. Therefore, it seems likely that these patients are examples of “false negative” PCR results that we encountered using PCR methodology at the time in more than 20% of Sézary patients with a chromosomally abnormal clone [27].-
 Table 4:Summary of flow cytometric results obtained by fine needle aspiration of lymph nodes from patients with mycosis fungoides and Sézary
syndrome. View Table
Table 4:Summary of flow cytometric results obtained by fine needle aspiration of lymph nodes from patients with mycosis fungoides and Sézary
syndrome. View Table
A box plot of CD4+CD7- and CD4+CD26- percentages according to absence or presence of detectable neoplastic cells by FNA is shown in Figure 2. For involved LNs, the percentage of CD4+CD26- cells (median, 57.5%) was higher than CD4+CD7- cells (median, 28.0%), but the difference was not statistically significant (P= 0.142). CD4+CD26- percentage was higher than CD4+CD7- in all but one case where CD4+CD7- cells were only 0.8% higher than CD4+CD26-. Consequently, the percentage of maxiCD4+CD7-/26- cells and CD4+CD26- cells was nearly identical and ranged between 1.5 to 93% (Table 4). All were above 20% except for a LN considered to be involved solely on the basis of a detectable T cell clone by PCR. Conversely, the 4 LNs without detectable neoplastic cells had maxiCD4+CD7-/26- percentages ranging from 5 to 25%.-
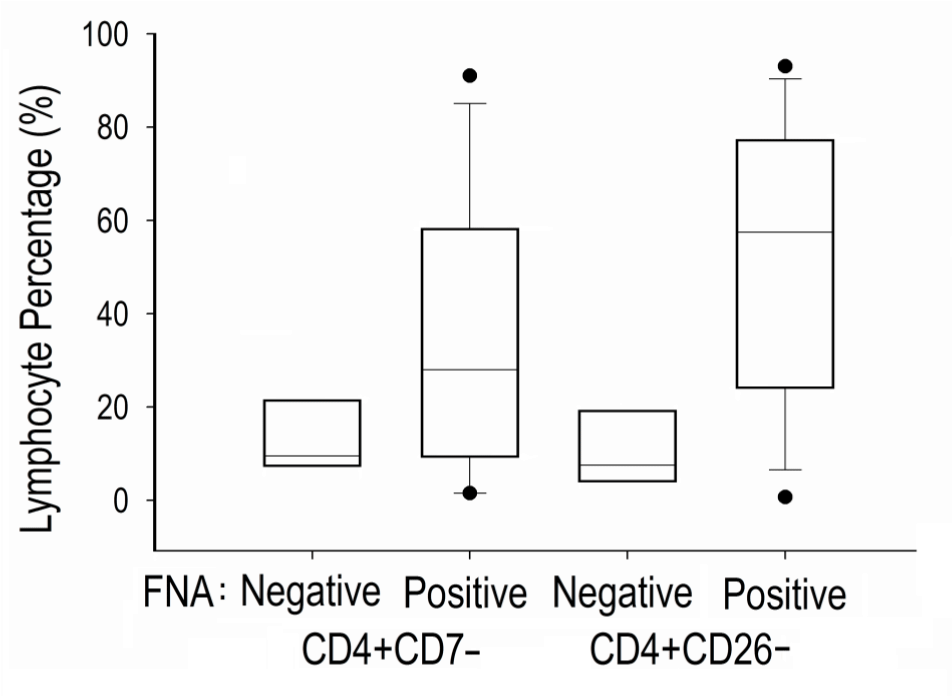 Figure 2: Median-quartile box plot of CD4+CD7- and CD4+CD26-
percentages according to absence or presence of detectable neoplastic
cells in 12 lymph nodes studied by fine needle aspiration (FNA) at the
time of initial staging. View Figure
Figure 2: Median-quartile box plot of CD4+CD7- and CD4+CD26-
percentages according to absence or presence of detectable neoplastic
cells in 12 lymph nodes studied by fine needle aspiration (FNA) at the
time of initial staging. View Figure
In terms of prognosis, the 13 patients with evidence of neoplastic cells in their LNs by FNA had a shorter mean DSS (5.40 years ±1.43) compared to the 4 patients without evidence of neoplastic cells (8.94 years ± 2.27); however, the difference was not statistically significant (P= 0.369). In the Cox model, for patients with LN involvement, maxi-CD4+CD7-/26- percentages provided a significant but only slightly better fit with disease-specific death as the endpoint than CD4+CD7- or CD4+CD26- alone whereas CD4/CD8 and CD4/ CD19 ratios were not significant. The mean DSS and 5-year survival rates for patients with maxi-CD4+CD7-/26- percentages in LNs ≥ 30%, ≥ 40% and ≥ 50% were 3.97 years/22%, 2.92 years/13% and 2.74 years/14%, respectively.The results of flow studies for involved LNs studied by FNA and involved LNs that were excised at the time of initial staging are compared in Supplemental Table 4. Altogether 13 of 17 (76%) LNs studied by FNA had evidence of neoplastic cells in the sample compared to 36 of 52 (69%) LNs excised at the time of initial staging. However, higher percentages of CD3+CD4+ T cells and lower CD19+ B cells were observed in FNA samples compared to excised LNs. Consequently, the calculated CD4/ CD19 ratio was also significantly higher in FNA samples (P< 0.001). These findings suggest that the LNs studied by FNA may have had a greater magnitude of involvement, i.e., were more effaced, than excised LNs. Although it is possible that FNA may have disproportionally sampled the paracortical zones of LNs where T cells predominant, the CD4/CD8 ratio and percentage of CD4+CD7- cells in FNA samples was only slightly higher than excised LNs.In this study, we attempted to identify flow parameters that might be used with FNA as surrogates for the LN4 histopathologic grade that is currently used for clinical staging of CTCL [3]. Potentially this goal might be attained by counting number of atypical cells per field on cytopathologic preparations [13], quantifying the number of clonal T cells by molecular methods [28], or determining percentage of lymphocytes with an abnormal immunophenotype by FC. The problem with counting atypical cells on cytopathologic preparations is that stimulated normal lymphocytes can develop hyperconvoluted “cerebriform” nuclei, thereby making distinction between neoplastic versus normal lymphocytes based on cell morphology alone difficult [29].Using outdated single parameter FC on excised LNs, we identified several criteria that had a similar DSS as LN4 nodes obtained at the time of clinical staging (shown in Table 3). Three of these criteria (CD4/CD8 ≥ 30, CD7+ cells ≤ 20% and CD4/ CD19 ≥ 8) were not positive in LNs graded as LN0-2, suggesting a possible role for diagnosis of involvement. However, only a minority of LN4 nodes fulfilled these criteria, indicating that a different approach was required for staging.In patients with CTCL, blood tumor burden has been estimated by the percentage of CD4+CD7- or CD4+CD26- cells [20-25]. Given that CD7 and CD26 expression by neoplastic T cells in a given case may be variable (some cells positive, some cells negative), it has been suggested that both populations be measured, and the maximum value used for determining blood tumor burden [26,27]. We now propose that a similar approach be applied to LNs studied by FNA.In this series, CD4+CD7- and CD4+CD26- lymphocytes were simultaneously measured in 18 LNs with evidence of neoplastic cells (6 LNs by excision, 12 LNs by FNA). The percentage of CD4+CD26- cells was higher than the percentage of CD4+CD7- for almost all cases. This is consistent with the observation that CD7 and CD26 expression by neoplastic T cells is lost by about 60% and 90% of patients with SS, respectively [22]. Consequently, the percentage of CD4+CD26- cells usually defines percentage of maxiCD4+CD7-/26- cells.Our data also suggest that patients with LNs that have percentages of maxiCD4+CD7-/26- ≥ 40% have a prognosis comparable to patients with effaced LN4 nodes and this might be used with FNA as a criterion for N3 node rating for clinical staging. However, we also observed that maxiCD4+CD7-/26- ≥ 40% occurred in 3 of 5 excised dermatopathic LNs without apparent histopathologic involvement. The CD4+CD26- percentages were 45%, 49% and 60% whereas the corresponding CD4+CD7- values were 14%, 23% and 27%. Accordingly, we propose that the threshold for maxiCD4+CD7-/26- as a surrogate for LN4 be increased to ≥ 50% plus evidence of involvement to minimize “false positives” until additional cases have been studied. The one patient with 60% CD4+CD26- cells had an LN3 node with high CD4/CD8 ratio (85%/5%).Of course, this proposed flow criterion might change as more LNs are studied. One confounding factor with measuring maxiCD4+CD7-/26- is that CD4 may not be expressed (CD4-CD8- or CD4-CD8+) by neoplastic T cells as occurred in 3 patients in this series or neoplastic cells might co-express CD7 and CD26 as occurred for one patient. Other markers of neoplastic cells such as antibodies directed against TCR-Vβ [30,31], Kir3DL2/CD158k [32,33] or CD164 [34] need to be investigated.The high percentage of CD71+ cells in involved LNs (median, 8.5%, range 1 to 56%) is consistent with a high proliferation rate of neoplastic cells. [35] However, high values of CD71 might be encountered in non-neoplastic hyperplastic LNs [36], and one of our LNs with follicular hyperplasia had 25% CD71+ cells plus evidence of a T cell clone. In future studies, it might be worth examining the diagnostic and prognostic implications of CD71 expressed on specific lymphocyte subsets, e.g., CD4+CD26-CD71+.Finally, in accordance with other studies [2,37,38], evidence of clear-cut histopathologic involvement in LNs was frequent in patients with advanced MF and SS and, if present at the time of staging, signifies a worse DSS compared to non-involved LNs for patients with MF and E-MF. However, for our patients diagnosed with SS, overt LN involvement was not associated with a significantly worse DSS compared to Sézary patients without involvement. We also did not find a significant difference in survival for nodal infiltrates composed predominately of small or large neoplastic cells as reported previously [11]. These findings suggest that pathologic assessment of LNs at the time of clinical staging may not be necessary for patients diagnosed as SS.The use of FNA to assess LNs in CTCL is controversial. One concern is that neoplastic involvement might be focal and thereby not be detected as compared to excision. The presence of nodal involvement often determines the approach to therapy for patients with MF, i.e., skin-directed only treatment versus skin treatment combined with systemic agent [39]. However, for clinical staging, the issue is also about whether the involved LN is partially or completely effaced (LN4 grade). We believe FNA combined with ancillary studies would identify involvement in such LNs.In conclusion, our data suggest that FNA coupled with measurement of CD4+CD7- and CD4+CD26- lymphocyte subsets and molecular analysis of T cell clonality might provide an alternative means to assess enlarged peripheral LNs in patients with CTCL, thereby decreasing the morbidity associated with excisional biopsies. A possible approach might be to initially perform FNA first and, if this is completely normal, then obtain an excisional biopsy if the clinical suspicion of nodal involvement is high. Prospective studies are required to determine the utility of this approach.Dr. Vonderheid organized the data, performed the statistical analysis and helped write the manuscript.Dr. Hou supervised some of the flow cytometry and helped write the manuscript.Dr. Bigler supervised some of the flow cytometry and helped write the manuscript. -
-
- Green SB, Byar DP, Lamberg SI. Prognostic variables in mycosis fungoides. Cancer. 1981; 47: 2671-2677.
- Sausville EA, Eddy JL, Makuch RW. Histopathologic staging at initial diagnosis of mycosis fungoides and the Sézary syndrome. Definition of three distinctive prognostic groups. Ann Intern Med. 1988;109: 372-382.
- Olsen E, Vonderheid E, Pimpinelli N. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007; 110: 1713-1722.
- Scheffer E, Meijer CJLM, Van Vloten WA. Dermatopathic lymphadenopathy and lymph node involvement in mycosis fungoides. Cancer. 1980; 45: 137-148.
- Matthews M, Gazdar A. Quoted in Clendenning WE, Rappaport HW. Report of the Committee on Pathology of Cutaneous T Cell Lymphomas. Cancer Treat Rep. 1979; 63: 719-724.
- Lynch JW Jr, Linoilla I, Sausville EA. Prognostic implications of evaluation for lymph node involvement by T-cell antigen receptor gene rearrangement in mycosis fungoides. Blood. 1992; 79: 3293-3299.
- Bakels V, Van Oostveen JW, Geerts ML, Gordijn RL, Walboomers JM, Scheffer E, et al. Diagnostic and prognostic significance of clonal T-cell receptor beta gene rearrangements in lymph nodes of patients with mycosis fungoides. J Pathol. 1993; 170: 249-255.
- Kern DE, Kidd PG, Moe R, Hanke D, Olerud JE. Analysis of T-cell receptor gene rearrangement in lymph nodes of patients with mycosis fungoides. Prognostic implications. Arch Dermatol. 1998; 134: 158-164.
- Juarez T, Isenhath SN, Polissar NL, Sabath DE, Wood B, Hanke D, et al. Analysis of T-cell receptor gene rearrangement for predicting clinical outcome in patients with cutaneous T-cell lymphoma: a comparison of Southern blot and polymerase chain reaction methods. Arch Dermatol. 2005; 141: 1107-1113.
- Fraser-Andrews EA, Mitchell T, Ferreira S, Seed PT, Russell-Jones R, Calonje E, et al. Molecular staging of lymph nodes from 60 patients with mycosis fungoides and Sézary syndrome: correlation with histopathology and outcome suggests prognostic relevance in mycosis fungoides. Br J Dermatol. 2006; 155: 756-762.
- Vonderheid EC, Diamond LW, van Vloten WA. Lymph node classification systems in cutaneous T-cell lymphoma. Evidence for the utility of the Working Formulation of Non-Hodgkin’s Lymphomas for Clinical Usage. Cancer. 1994; 73: 207-218.
- Assaf C, Hummel M, Steinhoff M, Geilen CC, Orawa H, Stein H, et al. Early TCR-beta and TCR-gamma PCR detection of T-cell clonality indicates minimal tumor disease in lymph nodes of cutaneous T-cell lymphoma: diagnostic and prognostic implications. Blood. 2005; 105: 503-510.
- Galindo LM, Garcia FU, Hanau CA, Lessin SR, Jhala N, Bigler RD, et al.Fine-needle aspiration biopsy in the evaluation of lymphadenopathy associated with cutaneous T-cell lymphoma (mycosis fungoides/Sézary syndrome). Am J Clin Pathol. 2000; 113: 865-871.
- Pai RK, Mullins FM, Kim YH. Cytologic evaluation of lymphadenopathy associated with mycosis fungoides and Sézary syndrome: role of immunophenotypic and molecular ancillary studies. Cancer. 2008; 114: 323-332.
- Vigliar E, Cozzolino I, Picardi M. Lymph node fine needle cytology in the staging and follow-up of cutaneous lymphomas. BMC Cancer. 2014; 14: 8.
- Vonderheid EC, Pena J, Nowell P. Sézary cell counts in erythrodermic cutaneous T-cell lymphoma: implications for prognosis and staging. Leuk Lymphoma. 2006; 47: 1841-1856.
- Vonderheid EC, Bigler RD, Kotecha A. Variable CD7 expression on T cells in the leukemic phase of cutaneous T cell lymphoma (Sézary syndrome). J Invest Dermatol. 2001; 117: 654-662.
- Collett D. Modelling Survival Data in Medical Research. London, UK: Chapman & Hall; 1997.
- Bhat R, Khandpur B, Vonderheid EC, Hou JS. FoxP3-positive T-regulatory cells in lymph nodes with mycosis fungoides and Sézary syndrome. Lymphoma. 2014; 597908: 9.
- Bernengo MG, Novelli M, Quaglino P, Lisa F, De Matteis A, Savoia P, et al. The relevance of the CD4+ CD26- subset in the identification of circulating Sézary cells. Br J Dermatol. 2001; 144: 125-135.
- Bogen SA, Pelley D, Charif M, McCusker M, Koh H, Foss F, et al. Immunophenotypic identification of Sézary cells in peripheral blood. Am J Clin Pathol. 1996; 106: 739-748.
- Vonderheid EC, Bernengo MG. The Sézary syndrome: hematologic criteria. Hematol Oncol Clin North Am. 2003; 17: 1367-1389.
- Novelli M, Fava P, Sarda C, Ponti R, Osella-Abate S, Savoia P, et al. Blood flow cytometry in Sézary syndrome: new insights on prognostic relevance and immunophenotypic changes during follow-up. Am J Clin Pathol. 2015; 143: 57-69.
- Vonderheid EC, Hou JS. CD4+CD26- lymphocytes are useful to assess blood involvement and define B ratings in cutaneous T cell lymphoma. Leuk Lymphoma. 2018; 59: 330-339.
- Scarisbrick JJ, Hodak E, Bagot M, Stranzenbach R, Stadler R, Ortiz-Romero PL, Papadavid E, et al. Blood classification and blood response criteria in mycosis fungoides and Sézary syndrome using flow cytometry: recommendations from the EORTC cutaneous lymphoma task force. Eur J Cancer. 2018; 93: 47-56.
- Vonderheid E. Comment on B ratings for erythrodermic cutaneous T-cell lymphoma. Eur J Cancer. 2018; 101: 281-283.
- Vonderheid EC. Prognostic Implications of Blood B Ratings for Erythrodermic Cutaneous T cell Lymphoma. J Clin Exp Dermatol Res. 2018; 9: 452-457.
- Mahe E, Pugh T, Kamel-Reid S. T cell clonality assessment: past, present and future. J Clin Pathol. 2018; 71: 195-200.
- Reinhold U, Herpertz M, Kukel S, Oltermann I, Uerlich M, Kreysel HW. Induction of nuclear contour irregularity during T-cell activation via the T-cell receptor/CD3 complex and CD2 antigens in the presence of phorbol esters. Blood. 1994; 83: 703-706.
- Feng B, Jorgensen JL, Jones D, Chen SS, Hu Y, Medeiros LJ, Wang SA. et al. Flow cytometric detection of peripheral blood involvement by mycosis fungoides and Sézary syndrome using T-cell receptor Vbeta chain antibodies and its application in blood staging. Mod Pathol. 2010; 23: 284-295.
- Gibson JF, Huang J, Liu KJ, Carlson KR, Foss F, Choi J, et al. Cutaneous T-cell lymphoma (CTCL): Current practices in blood assessment and the utility of T-cell receptor (TCR)-Vβ chain restriction. J Am Acad Dermatol. 2016; 74: 870-877.
- Moins-Teisserenc H, Daubord M, Clave E. CD158k is a reliable marker for diagnosis of Sézary syndrome and reveals an unprecedented heterogeneity of circulating malignant cells. J Invest Dermatol. 2015; 135: 247-257.
- Battistella M, Leboeuf C, Ram-Wolff C. KIR3DL2 expression in cutaneous T-cell lymphomas: expanding the spectrum for KIR3DL2 targeting. Blood. 2017; 130: 2900-2902.
- Guenova E, Ignatova D, Chang YT, Contassot E, Mehra T, Saulite I, et al. Expression of CD164 on Malignant T cells in Sézary Syndrome. Acta Derm Venereol. 2016; 96: 464-467.
- Trowbridge IS, Omary MB. Human cell surface glycoprotein related to cell proliferation is the receptor for transferrin. Proc Natl Acad Sci. 1981; 78: 3039-3043.
- Self SE, Burdash NM, Ponzio AD. Lymphocyte subsets in lymph node hyperplasias and B cell neoplasms as determined by fluoresceinated antibodies and flow cytometry. Ann N Y Acad Sci. 1986; 468: 195-210.
- Agar NS, Wedgeworth E, Crichton S, Mitchell TJ, Cox M, Ferreira S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organization for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010; 28: 4730-4739.
- Alberti-Violetti S, Talpur R, Schlichte M. Advanced-stage mycosis fungoides and Sézary syndrome: survival and response to treatment. Clin Lymphoma Myeloma Leuk. 2015; 15: e105-112.
- Trautinger F, Eder J, Assaf C, Bagot M, Cozzio A, Dummer R, et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome - Update 2017. Eur J Cancer. 2017; 77: 57-74.