Product |
Brand Name |
Ingredients |
Claim |
1 |
Prime Labs Men’s Testosterone Booster |
Tongkat Ali Extract, saw palmetto extract, orchic substance wild yam extract, sarsaparilla, nettle extract, boron, magnesium stearate, silicon dioxide, hydroxypropyl methylcellulose, microcrystalline cellulose, stearic acid |
|
2 |
Leyzene |
Icariin, arginine HCl, royal jelly, ginseng, black pepper extract, gluconolactone, magnesium sterate, and gelatin Capsule |
|
3 |
Horny Coat Weed Herbal Complex |
Horny Goat Weed extract, maca root, tongkat Ali, ginseng, L-Arginine |
|
4
|
Organic Maca Root |
Organic Maca Root, Black Pepper Extract, Vegetable Cellulose Capsule |
|
5 |
AlphaMan XL |
Macuna Pruriens (L-Dopa), Polypodium Vulgare, Yohimbe Bark, Saw Palmetto, Muira Puama, L-Arginine & Panax Ginseng |
|
6 |
Golden X |
zinc, maca root, horny goat weed, ginseng, Tribulus terrestris and pomegranate, Schisandra Berry |
|
7 |
BigRize |
boosts testosterone, fast and long acting |
|
8 |
boostULTIMATE Testosterone Booster Pills |
Ginseng, Muira Puma, and Maca, L-arginine |
|
9 |
Boost Elite Test Booster |
Tribulus Terrestris Extract, Horny Goat Weed, Fenugreek Extract, Maca Root, Zinc Citrate, DIM, Panax Ginseng, Tongkat Ali Extract, & Yohimbe Bark Extract |
|
10 |
High T Black Natural Testosterone Booster |
Nitric oxide, arginine Alpha-Ketoglutarate, citrulline-malate, beta alanine, caffeine anhydrous, eurycoma longifolia, raspberry ketone, rhodiola rosea, and testofen fenugreek extract. |
|
Journal of Sexual Medicine and Reproductive Health
-
Mini ReviewAre Male Enhancement Ultraceuticals Sold Online Safe, Natural and Compliant with their Label Claim?Nadine Amine, Cindy Zheng, Harshvir Kaur, Jessica Sweeney, Mark Mikail, and Mohamed Ismail Nounou*Department of Pharmaceutical Sciences, University of Saint Joseph, USA*Corresponding author: Mohamed Ismail Nounou, Department of Pharmaceutical Sciences (DPS), School of Pharmacy and Physician Assistant Studies (SOPPAS), University of Saint Joseph (USJ), Hartford, CT, 06103, USA, Email: nounou@usj.eduSubmitted: 30 May 2019; Accepted: 16 June 2019; Published: 18 June 2019
-
Attention to ultraceuticals has become extremely prevalent. A large surge of male enhancement ultraceuticals hashit the U.S. market through online retail stores such as eBay, Amazon, GNC, and Alibaba that consist of unverified therapeutic label claims. The issue at hand is the increase in the production, marketing and sale of adulterated products from these online retail stores that falsify the safety, efficacy, and therapeutic claims on their websites. These products are further provoked to continue their false claims due to the absence of strict regulatory laws to govern label claims, manufacturing, and marketing in U.S. and global markets. The online retail stores that sponsor and advertise the sales of these ultraceuticals neglect the literature that show many of the products were discovered to be counterfeit, mislabeled, or adulterated with active pharmaceutical ingredients (API). Ultimately, regulatory authorities should instill regulations in order to monitor, examine, and test the ultraceutical market and the research community should draw public attention to the possible effects of these products on consumers.Keywords: Ultraceuticals; Adulteration; Counterfeit; Nutraceuticals; Male enhancement; Online retail stores
-
Natural, organic, chemical free, and homeopathic, are all buzzwords consumers tend to associate with safety and efficacy. In many cases, such marketing terminology is misleading and deceitful [1]. Many people seek out this terminology when purchasing dietary supplements and vitamins. Health organizations and researchers have attempted to introduce policies to regulate these industries and have largely failed due to a lack of interest [2-5]. Ultraceutical formulations are expanding widely to include products that include male-enhancement, weight loss, and cognitive enhancement products. The U.S. Food and Drug Administration (FDA) have very limited control over dietary supplements. The FDA only regulates the labeling of these products in the United States, requiring a disclaimer label stating “This statement has not been evaluated by the FDA. This product is not intended to prevent, cure or treat any disease” [6]. Despite this, the advertised claims, trade names, and even the shape of the pills suggest therapeutic effects as seen on their online retail stores.Online sale of Ultraceuticals: a market place uncheckedThe absence of regulatory laws, enforcement, and oversight of the ultraceutical industry promotes the production and adulteration of the products being sold; thus, encouraging their trade throughout online retail stores including Alibaba, GNC, eBay, and Amazon [7-10]. This is a major area of sales for unregulated ultraceutical sales in the United States. In March 2019, over 10,000 and 10,938 male enhancement nutraceuticals were advertised on Amazon and eBay, respectively [8,9]. Tables 1-4 list the top ten male enhancement ultraceuticals sold online on Alibaba, GNC, eBay, and Amazon [7-10]. Comparing the four markets, it is observed that the label on the products contains the required FDA disclaimer label. However, in the title and description of the products is where consumers will find many unverified therapeutic claims. On Alibaba and eBay [7,9] (Tables 2,4) claims are aggressive, stating they are 100% safe, natural, and of high quality with zero additives, as seen on the labels. Such claims do not comply with the FDA labeling requirements for dietary supplements [11]. In case of Alibaba and eBay [7,9] many ingredients are not listed in the description and many omit the ingredients label on the bottle. As for Amazon [8] (Table 1), most products are claimed to be natural, however, the ingredients are clearly shown in the product description. As for General Nutrition Centers (GNC) (Table 3), ingredients and uses are clearly stated in the description with clear ingredient labels and pictures of the product. All these male enhancement ultraceuticals sold online have therapeutic claims, such as boosting testosterone, increase strength, or increase sexual health [10]. Table 1 Ten top-selling products of male enhancement ultraceuticals on Amazon and eBay retail online stores (February 2019).
-
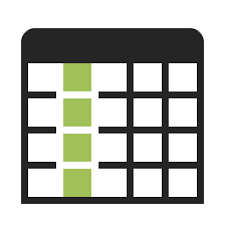 Table 1:Ten top-selling products of male enhancement ultraceuticals on Amazon retail online store in the United States (February 2019)
[8]. View Table
Table 1:Ten top-selling products of male enhancement ultraceuticals on Amazon retail online store in the United States (February 2019)
[8]. View Table
-
 Table 2:Ten top-selling products of male enhancement ultraceuticals on eBay online store in the United States (February 2019) [9]. View Table
Table 2:Ten top-selling products of male enhancement ultraceuticals on eBay online store in the United States (February 2019) [9]. View Table
-
 Table 3:Ten top-selling products of male enhancement ultraceuticals on General Nutrition Centers (GNC) online and retail stores in the United States
(February 2019) [10]. View Table
Table 3:Ten top-selling products of male enhancement ultraceuticals on General Nutrition Centers (GNC) online and retail stores in the United States
(February 2019) [10]. View Table
-
 Table 4:Ten top-selling products of male enhancement ultraceuticals on the global Alibabaonline store (February 2019) [7]. View Table
Table 4:Ten top-selling products of male enhancement ultraceuticals on the global Alibabaonline store (February 2019) [7]. View Table
Contents and ConcernsUltraceuticals are not all safe, despite common misconception. This can be attributed to their unnecessary use, unknown ingredients, and low pharmaceutical quality [1,12-16]. Some products in the ultraceuticals category have been found to contain pesticides, heavy metals, and adulterants [17-20]. Adulterated products may contain incorrect parts of a plant or unreported active pharmaceutical ingredients (APIs), as documented in previous cases [12,17-20]. Herbal adulteration has also been a problem. A recent study found male enhancement supplements contained at least 1 of 5 unreported plants of concern [21]. Other cases of adulteration of male-enhancement nutraceuticals with sildenafil, vardenafil, or tadalafil have been previously reported [12]. Fraudulent adulteration may increase the therapeutic effect but is illegal, unethical, and can cause significant harm. Following consumption of the male enhancement supplement Rhino 7 Platinum 3000, a 25-year-old man required bilateral corpoglanular shunting to alleviate his priapism that had persisted for 48 hours; A follow up appointment demonstrated he is no longer able to maintain an erection and has significant bilateral fibrosis of the corporal bodies [22].The purpose of the undocumented APIs spiking and adulteration is for manufacturers to increase the therapeutic effects of their products; simultaneously claiming them to be of natural origin in order to market their products in a more favorable, pleasing psychological influence to consumers [17- 20]. Consumers are unaware of the true ingredients within these naturally claimed products, which make them susceptible to drug-drug interactions that can cause a great risk to their health and safety. It can ultimately cause permanent damage as seen in the case of Rhino 7 Platinum 3000 [22].Many of the male enhancement ultraceuticals sold online were found to be adulterated with reported or unreported analogues of FDA-approved prescription phosphodiesterase-5 inhibitors (PDE5i) used to treat erectile dysfunction such as sildenafil, tadalafil, or vardenafil [1,11,12,15,16]. A study conducted by Pfizer Global Security and Research and Development teams found 81% of sexual performance ultraceuticals contained adulterated PDE5is, 45% of those contained more than the highest approved strength of the drug [23]. Novel PDE5i analogues such as desmethylpieprazinyl propoxysildenafil continue to be discovered in this class of dietary supplements [24]. The presence and quantity of these adulterants is of great concern due to the known risks associated with this class of FDA approved drugs and the uncertainty of the safety of the untested PDE5i analogues. ZenMaxx, Instant Hard Rod, and RigiRx Plus were all found to contain amino-tadalal, an analog of tadalafil [25]. This interacts with nitrates found in some prescription drugs and may dangerously lower blood pressure [5,26,27]. These products pose a threat to patients with diabetes, high blood pressure, high cholesterol, or heart disease [28]. Many of the products described above can be seen on the FDA list of tainted products marketed as dietary supplements though, based on a survey of current literature, however, it is by no means a comprehensive list [11].Action requiredNutraceuticals and ultraceuticals are advertised as purely natural products free from side effects. They are widely sold online via major retail stores such as Amazon, eBay, and Alibaba from unknown sources from all over the world, mostly with a therapeutic claim that violates FDA and DSHEA guidelines [6- 9]. Literature review shows that these products are adulterated, misbranded, mislabeled, are of low pharmaceutical quality, and may be dangerous because of the presence of undeclared plant products and APIs. These products often contain disingenuous review from which consumers base their decision making [29]. Health organizations and governments are aware of the illegal manufacture and sale of adulterated and counterfeit nutraceutical formulations, yet no laws or regulations are enforced to tackle this global problem [30].There are expanding issues around adulterated and counterfeit nutraceuticals globally. There seems to be gray areas in the U.S. market where these nutraceuticals are being sold regarding their claim, safety, efficacy, registration, sales, and regulation.Male enhancement nutraceuticals which are sold freely online should be researched, analyzed, and evaluated with respect to claim, content, safety, and efficacy. Researchers should be encouraged to investigate the quality, safety, and efficacy of supplements suspected to have illegal therapeutic claims. Technologies should be utilized for the fast and accurate detection of adulterants and counterfeit products. These technologies include near infra-red (NIR) spectrophotometry or simple spectrophotometric analysis [1,31,32]. This problem has led to the development of innovative detection technologies including portable drug identification devices such as Si-Ware systems fully integrated chip-sized spectral sensor (NeoSpectra™ sensors) and Stratio LinkSquare pen spectrophotomer for industrial and consumer markets [31,32]. NeoSpectra™ sensors are compact and low-cost Fourier Transform near InfraRed (FT-NIR) spectral sensors that can provide portable, fast and accurate analyses for medical and pharmaceutical application. Such systems can be used for the fast and accurate detection of adulterants and counterfeit products in the pharmaceutical industry sector, insurance agencies, law enforcement agencies, airports and retail pharmacies along with consumers [32]. They deliver the spectral response of the light absorbed by materials for quantification, qualification or identification [32]. Moreover, the development of rapid and accurate analytical techniques is critical. A new assay has been developed to rapidly detect PDE5 inhibitors using fluorescence that can even successfully uncover new analogues in low concentrations [33]. Ultraceuticals research is critical for online marketplace warehouses, regulatory agencies, and consumers with respect to safety, efficacy and quality.Ultimately, this problem is the result of a rapidly growing industry going unchecked. Global and national health organizations should implement new regulations or enforce existing regulations for nutraceuticals to protect consumers and hold those who profit from their manufacture, distribution, and sale accountable for adverse effects. -
-
- ElAgouri G, ElAmrawy F, ElYazbi A, Eshra A, Nounou MI. Male enhancement Nutraceuticals in the Middle East market: Claim, pharmaceutical quality and safety assessments. Int J Pharm. 2015; 492: 109-119.
- Defelice SL. FIM Rationale and Proposed Guidelines for the Nutraceutical Research & Education Act. NREA. Presented at FIM's 10th Nutraceutical Conference November 10-11, 2002; The Waldorf-Astoria, New York City. 2002.
- United Arab Emirates Ministry of Health. Ministry of Health warns about online circulation of medicines UAE: Emirates news agency (WAM); 2016.
- Heyland DK. In search of the magic nutraceutical: problems with current approaches. J Nutr. 2001; 131: 2591-2595.
- S. Food and Drug Administration (FDA). Label claims for conventional foods and dietary supplements. In: U.S. Food and Drug Administration (FDA), editor. Silver Springs (MD): U.S. Food and Drug Administration (FDA); 2018.
- Dickinson A. History and overview of DSHEA. Fitoterapia. 2011; 82: 5-10.
- Alibaba Online Store: Alibaba, Inc. 2019. Alibaba international retail store.
- Amazon Inc. Amazon Online Store: Amazon Inc. 2019.
- eBay Inc. eBay Online Store: eBay Inc. 2019.
- General Nutrition Centers (GNC). General Nutrition Centers (GNC) Retail & Online Stores: General Nutrition Centers (GNC), Inc; 2019.
- S. Food and Drug Administration (FDA). Tainted Products Marketed as Dietary Supplements. In: Department of Health and Human Services, editor. Maryland, USA: U.S. Food and Drug Administration (FDA); 2019.
- ElAmrawy F, ElAgouri G, Elnoweam O, Aboelazayem S, Farouk E, Nounou MI. Adulterated and Counterfeit Male Enhancement Nutraceuticals and Dietary Supplements Pose a Real Threat to the Management of Erectile Dysfunction: A Global Perspective. J Diet Suppl. 2016; 13: 660-693.
- Haller C, Kearney T, Bent S, Ko R, Benowitz N, Olson K. Dietary supplement adverse events: report of a one-year poison center surveillance project. J Med Toxicol. 2008; 4: 84-92.
- Petroczi A, Taylor G, Naughton DP. Mission impossible? Regulatory and enforcement issues to ensure safety of dietary supplements. Food Chem Toxicol. 2011; 49: 393-402.
- Ahmed N, Nounou MI, Abouelfetouh A, El-Kamel A. Over-the-counter Weight-Loss Herbal Supplements in Egypt: Label Claim, Microbiological, Pharmaceutical Quality and Safety Assessments. Med Princ Pract. 2018.
- Nounou MI, El Haddad G, El Amrawy F, El Gaddar O, El Yazbi A, Eshra A. Comparative Randomized Crossover Clinical Study for the Evaluation of Erectile Dysfunction Medications Via Novel Pentagon System. Curr Drug Saf. 2018; 13: 12-20.
- Van Breemen RB, Fong HH, Farnsworth NR. The role of quality assurance and standardization in the safety of botanical dietary supplements. Chem Res Toxicol. 2007; 20: 577-582.
- Kennedy DA, Seely D. Clinically based evidence of drug-herb interactions: a systematic review. Expert Opin Drug Saf. 2010; 9: 79-124.
- Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs: an updated systematic review. Drugs. 2009; 69: 1777-1798.
- Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs: a systematic review. Drugs. 2001; 61: 2163-2175.
- Deconinck E, Vanhamme M, Bothy JL, Courselle P. A strategy based on fingerprinting and chemometrics for the detection of regulated plants in plant food supplements from the Belgian market: Two case studies. J Pharm Biomed Anal. 2019; 166: 189-196.
- Mittakanti HR, Elliott CS. Priapism caused by "Rhino 7 Platinum 3000" an over-the-counter male enhancement supplement. Int J Impot Res. 2018; 30: 190-191.
- Campbell N, Clark JP, Stecher VJ, Thomas JW, Callanan AC, Donnelly BF, et al. Adulteration of purported herbal and natural sexual performance enhancement dietary supplements with synthetic phosphodiesterase type 5 inhibitors. J Sex Med. 2013; 10: 1842-1849.
- Lee JH, Park HN, Jung A, Mandava S, Park S, Lee J, et al. Isolation and characterisation of a novel sildenafil analogue adulterant, desmethylpiperazinyl propoxysildenafil, in a dietary supplement. Sci Justice. 2018; 58: 447-454.
- S. Food and Drug Administration (FDA). Public notification: “Instant Hard Rod” contains undeclared drug ingredient. In: U.S. Food and Drug Administration (FDA), editor. Silver Springs (MD): US Food and Drug Administration (FDA); 2016.
- S. Food and Drug Administration (FDA). Recall: Bethel nutritional consulting, inc. issues a voluntary recall of weight loss pills “Bethel 30” found to contain an undeclared drug ingredient. In: Department of Health and Human Services, editor. Maryland, U.S.A: US Food and Drug Administration (FDA); 2013.
- S. Food and Drug Administration (FDA). Enhance Nutraceutical 7/15/13. In: US Food and Drug Administration (FDA), editor. Silver Springs (MD): U.S. Food and Drug Administration (FDA); 2013.
- Health Canada. Recalls and safety alerts: Instant Hard Rod, RigiRx Plus, ZenMaxx. In: Health Canada, editor. Ottawa, Ontario (Canada): Health Canada; 2012.
- Balasubramanian A, Thirumavalavan N, Srivatsav A, Yu J, Lipshultz LI, Pastuszak AW. Testosterone Imposters: An Analysis of Popular Online Testosterone Boosting Supplements. J Sex Med. 2019; 16: 203-212.
- Cockburn R, Newton PN, Agyarko EK, Akunyili D, White NJ. The global threat of counterfeit drugs: why industry and governments must communicate the dangers. PLoS Med. 2005; 2: e100.
- Stratio Inc. Broadband, handheld and affordable spectrometer (Linksquare) for fast and reliable drug identification San Jose, CA: Stratio, Inc; 2019.
- Si-Ware Systems (SWS). Si-Ware systems introduces first fully integrated chip-sized spectral sensor for industrial and consumer markets Cairo, Egypt: Si-Ware Systems (SWS); 2019.
- Santillo MF, Mapa MST. Phosphodiesterase (PDE5) inhibition assay for rapid detection of erectile dysfunction drugs and analogs in sexual enhancement products. Drug Test Anal. 2018.