Case Report
A Case Report of Aortic Thrombus as a Result of Plasminogen Activator Inhibitor-1 Elevation in a Patient with Splenic Infarction
Chompunut Asavaaree1, Adam Dahlen DO2, Antony D Rawindraraj2, and Saksith Smithason3*
1Department of Cardiology, Southeastern Regional Medical Center, USA
2Department of Hospital Medicine, Southeastern Regional Medical Center, USA
3Department of Surgery, Southeastern Regional Medical Center, USA
*Corresponding author: Saksith Smithason, Department of Surgery,
Southeastern Regional Medical Center, 300 W 27th St, Lumberton, NC, USA, Tel: 910-674-2625; Email:
smithason4089@gmail.com
Submitted: 12 February 2019; Accepted: 17 April 2019; Published: 19 April 2019
Cite this article: Asavaaree C, Adam Dahlen DO, Rawindraraj AD, Smithason S
(2019) A Case Report of Aortic Thrombus as a Result of Plasminogen Activator Inhibitor-1 Elevation in a Patient with Splenic Infarction. JSM Vasc Med Res 4: 3.
We reported the case of abdominal aortic thrombus as a result of plasminogen activator inhibitor-1 (PAI-1) elevation. The patient was
a 65-year-old female who presented with acute left upper quadrant abdominal pain from splenic embolic infarction. A mural thrombus was
found in the abdominal aorta rostral to the origin of the celiac and splenic artery. The patient was in a thrombophilic state from elevation of
PAI-1. Unfractionated heparin bridge to coumadin resulted in complete resolution of the thrombus within 3 months of treatment.
There are 3 steps in the formation and degradation of a fibrin
thrombus. First, formation and stabilization, second, inhibition of
fibrinolysis, and third, activation of fibrinolysis [1]. Fibrinolysis
is balanced by factors of activation and inactivation (inhibitor).
Tissue plasminogen activator (t-PA) activates fibrinolysis and
is protected from inactivation by its inhibitor, the plasminogen
activator inhibitor-1 (PAI-1) [1]. Literature has shown ischemic
cardiovascular events from increased plasma PAI-1 [2], whereas
deficiency of PAI-1 results in severe bleeding [3]. Increased
PAI-1 is associated with many factors including inflammatory
cytokines, interleukins, growth factors, insulin resistance,
bacterial endotoxins, race, ethnicity, gender, increased BMI,
genetics, stress response, and smoking [4]. We present a patient
with embolic splenic infarction as a result of aortic thrombus due
to PAI-1 elevation.
The patient was a 65-year-old white female who presented
with a three day history of left upper quadrant abdominal
pain, worsening after deep breathing. She had a past history of
unprovoked pulmonary embolism while taking contraceptive
pills 15 years prior, was treated with intravenous unfractionated heparin, and then transitioned to coumadin for a total duration of
6 months. She also had a history of asthma, gout, and hypertension.
She had an up-to-date mammogram, Pap smear, and colonoscopy.
Her initial vitals were normal with a temperature 37.2 Celsius,
blood pressure 131/70 mmHg, respiratory rate 18 per minute,
heart rate 75 per minute, and her BMI was 26 kg/m2.There was
point tenderness at the left upper abdominal quadrant. Bowel
sounds were normal and there was no pleural rub. Admission labs
including complete blood count, blood chemistry, and urinalysis
were unremarkable. Stat abdominal contrast CT revealed focal
segmental splenic infarction (Figure 1) and abdominal aortic
thrombus proximal to the take-off of the celiac artery, measuring
2x4 cm in size (Figure 2). At this point, embolic splenic infarction
from the aortic thrombus was diagnosed. Further investigations
to locate the anatomical sources of the aortic thrombus were
negative. Neither thrombus nor embolic origin was found on CTAgated
aorta. Transthoracic echocardiogram and echocardiogram
with bubble study were negative for intracardiac thrombus,
vegetation or patent foramen ovale. Blood cultures were
negative for bacteremia. Exploring the infectious cause of
splenic infarction, along with hematologic malignancy causes
was negative (Cytomegalovirus titer, Human Immunodeficiency
Virus antigen, JAK2 mutation, Serum and Urine Protein
Electrophoresis). She had no prior recent abdominal trauma and
no recent abdominal surgery. Thrombophilia profile including
protein C, protein S, factor V Leiden, antiphospholipid, lupus
anticoagulant, prothrombin gene mutation, beta-2 glycoprotein,
lipoprotein, homocysteine, and fibrinogen were within normal
range, except the elevated PAI-1 level of 90 ng/mL (range 4-43
ng/mL, enzyme immunoassay (EIA), Diagnostic Nichols Institute,
Capistrano, CA, USA). Provided that no surgery was warranted
from general surgeon consultation, unfractionated heparin
drip with venous thromboembolism protocol was immediately
initiated with a target factor Xa level of 0.3-0.7 IU/mL. The patient
was ultimately bridged to coumadin with a target INR 2-3. She
promptly reported less abdominal pain within the first 24 hours.
Intravenous morphine was discontinued at 48 hours. Follow up
contrast abdominal CT at day 6 showed recanalization of the aorta (Figure 3). At 3 months follow up, contrast abdominal CT
displayed complete resolution of the aortic thrombi (Figure 4).
The spleen receives 5% of the cardiac output making it
prone to ischemic infarction [5]. From an ante mortem series,
Frippiat et al., reviewed 64 cases of splenic infarction and
found hematologic disorder as a leading etiology [6], whereas
Schatter et al., found cardiogenic emboli was the predominant
etiology [5]. Schatter et al., also described an autoimmune
disease, infection, and hematologic malignancy as the etiologies
of splenic infarction from their 32 case series. From the post
mortem study, Okefee et al., found embolic source was the most
common etiology in their 96 autopsies [7]. The same study found splenic congestion and splenic vein thrombosis were the
most common causes of non-embolic source. Taken together,
cardiogenic emboli and hematologic disease are among the most
common etiology of splenic infarction. Provided a broad range
of etiologies, splenic infarction could is the first presentation
of a significant underlying disease in an otherwise previously
healthy patient [5]. In this case, we explored all the possible
causes of splenic infarction as described in the literature. Embolic
cause of splenic infarction was established after we discovered
thrombus in her abdominal aorta. Further investigation
elucidated the thrombophilic state after we uncovered an
elevation of PAI-1. To the best of our knowledge, only one case
of splenic infarction from aortic thrombus has been previously
reported, but not definitively identifying an underlying etiology [8]. We therefore report the unique case of aortic thrombus, as
a result of increasing PAI-1 activity, leading to splenic infarction.
Regarding the treatment of aortic thrombus, thrombectomy or
anticoagulation, no standard guideline has been published yet.
The optimal duration of therapeutic anticoagulation is still less
defined in the literature. Verma et al., found association between
hyper coagulation and occult malignancy in their 88 patients with
aortic thrombus who developed either limb or visceral ischemia
[9]. All of their hypercoagulable patients were prescribed lifelong
therapeutic anticoagulation. Bowdish et al., reported 5 cases with
aortic mural thrombus from a hypercoagulable state, all treated
with therapeutic anticoagulation [10]. Given its simplicity and
familiarity, we chose unfractionated intravenous heparin, venous
thromboembolism protocol bridging to coumadin with target
INR 2-3 in this case. The success of the treatment is attested
by complete resolution of aortic thrombus at 3 months follow
up CT scan. Given this was her second episode of unprovoked
thrombosis, the patient agreed upon long-term therapeutic
anticoagulation. In summary, our case not only demonstrated a
clinical presentation and diagnosis of splenic infarction as a result
of thrombophillia, but we also demonstrated the effectiveness of
anticoagulation protocol in dissolving aortic thrombus from PAI-
1 elevation.
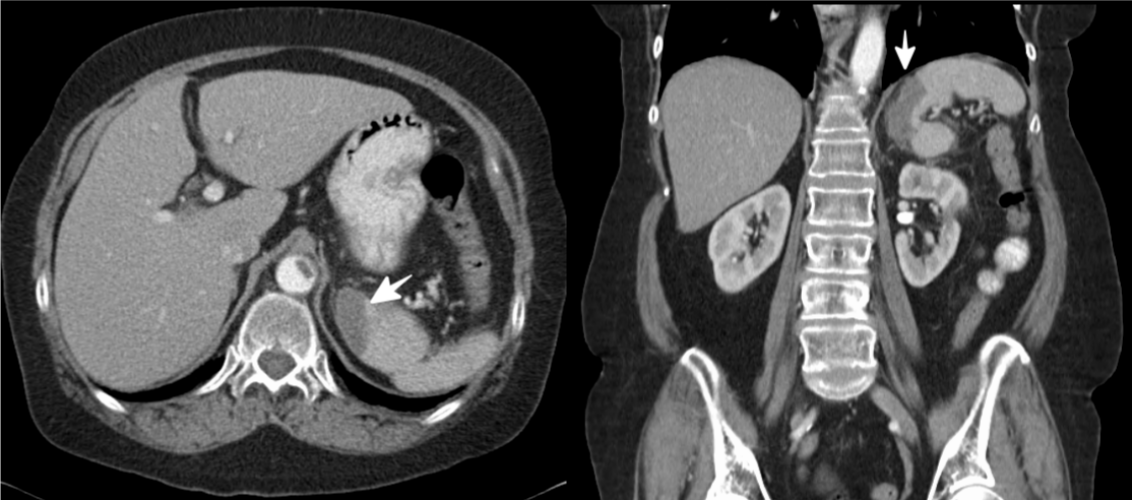
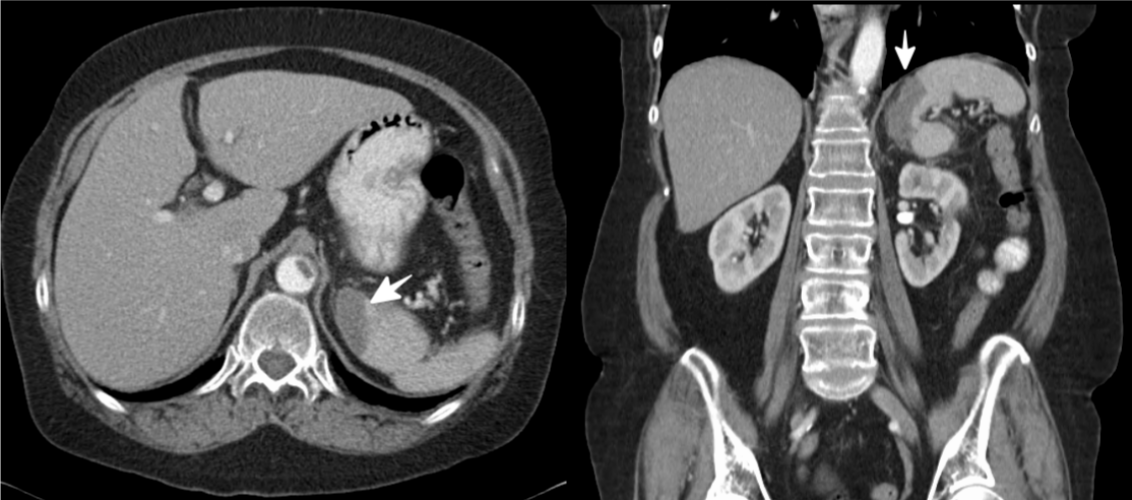 Figure 1:Splenic infarction (white arrow). View Figure
Figure 1:Splenic infarction (white arrow). View Figure
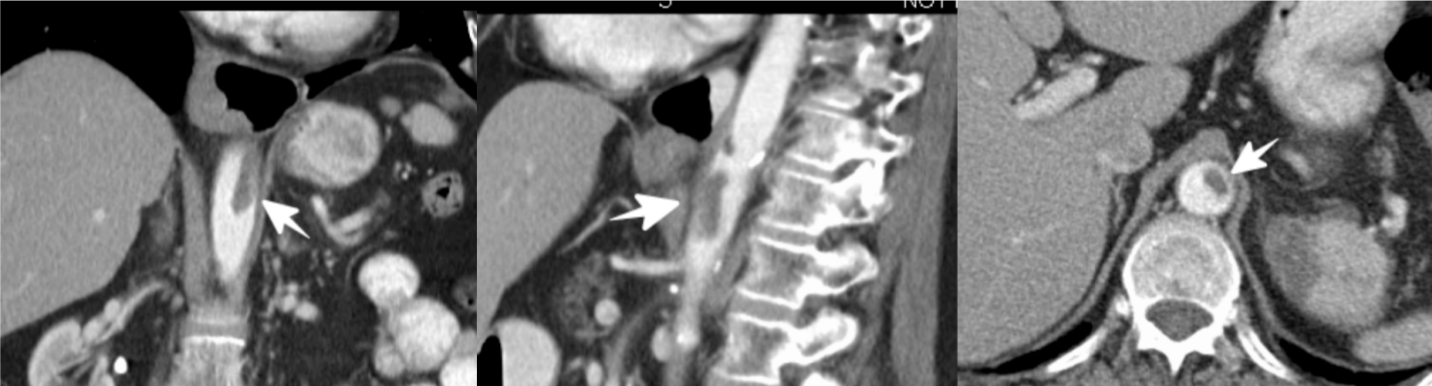 Figure 2:Abdominal aortic thrombus (white arrow) displayed on admission CT abdomen. View Figure
Figure 2:Abdominal aortic thrombus (white arrow) displayed on admission CT abdomen. View Figure
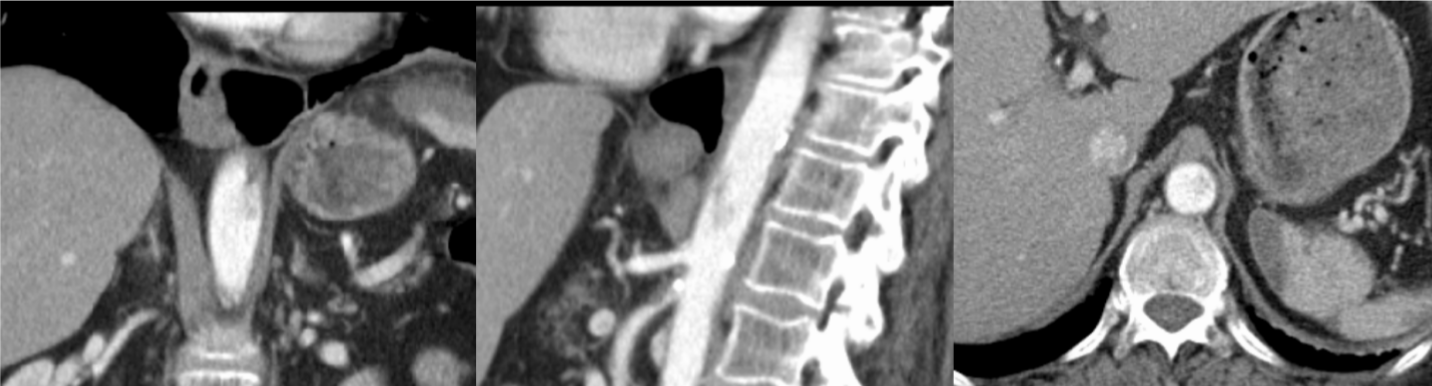 Figure 3:Partial dissolution of the thrombus after 5 days of treatment. View Figure
Figure 3:Partial dissolution of the thrombus after 5 days of treatment. View Figure
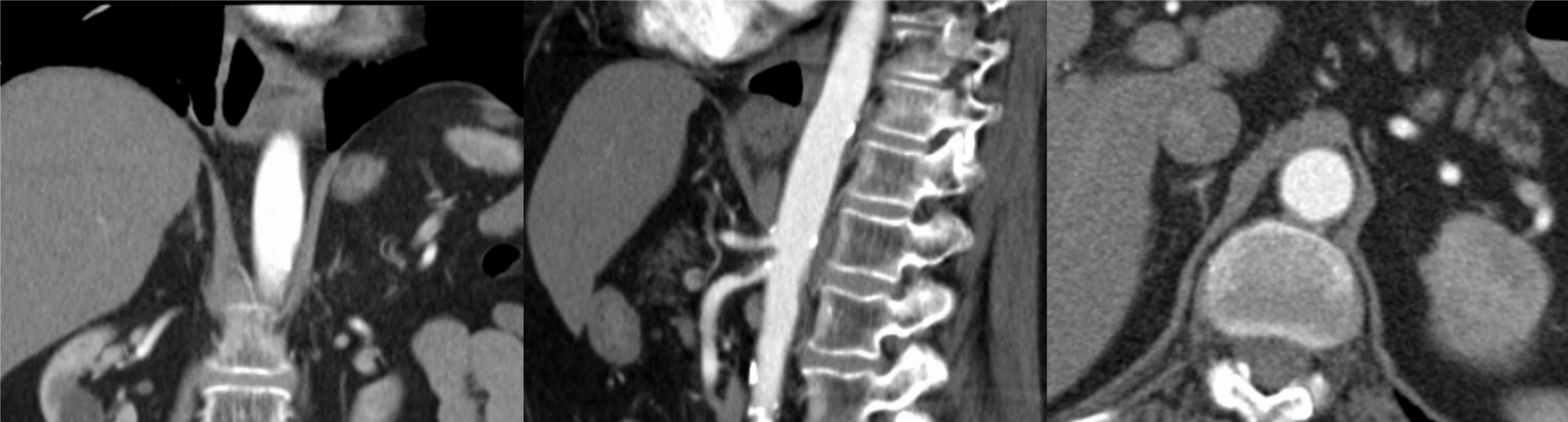 Figure 4:Complete dissolution of the thrombus after 3 months. View Figure
Figure 4:Complete dissolution of the thrombus after 3 months. View Figure